Abstract
Background:
Obesity is associated with low-grade systemic inflammation, in part because of secretion of proinflammatory cytokines, resulting into peripheral insulin resistance (IR). Increased oxidative stress is proposed to link adiposity and chronic inflammation. The effects of endurance exercise in modulating these outcomes in insulin-resistant obese adults remain unclear. We investigated the effect of endurance exercise on markers of oxidative damage (4-hydroxy-2-nonenal (4-HNE), protein carbonyls (PCs)) and antioxidant enzymes (superoxide dismutase (SOD), catalase) in skeletal muscle; urinary markers of oxidative stress (8-hydroxy-2-deoxyguanosine (8-OHdG), 8-isoprostane); and plasma cytokines (C-reactive protein (CRP), interleukin-6 (IL-6), leptin, adiponectin).
Methods:
Age- and fitness-matched sedentary obese and lean men (n=9 per group) underwent 3 months of moderate-intensity endurance cycling training with a vastus lateralis biopsy, 24-h urine sample and venous blood samples taken before and after the intervention.
Results:
Obese subjects had increased levels of oxidative damage: 4-HNE (+37%; P⩽0.03) and PC (+63%; P⩽0.02); evidence of increased adaptive response to oxidative stress because of elevated levels of copper/zinc SOD (Cu/ZnSOD) protein content (+84%; P⩽0.01); increased markers of inflammation: CRP (+737%; P⩽0.0001) and IL-6 (+85%; P⩽0.03), and these correlated with increased markers of obesity; and increased leptin (+262%; P⩽0.0001) with lower adiponectin (−27%; P⩽0.01) levels vs lean controls. Training reduced 4-HNE (−10%; P⩽0.04), PC (−21%; P⩽0.05), 8-isoprostane (−26%; P⩽0.02) and leptin levels (−33%; P⩽0.01); had a tendency to decrease IL-6 levels (−21%; P=0.07) and IR (−17%; P=0.10); and increased manganese SOD (MnSOD) levels (+47%; P⩽0.01).
Conclusion:
Endurance exercise reduced skeletal muscle-specific and systemic oxidative damage while improving IR and cytokine profile associated with obesity, independent of weight loss. Hence, exercise is a useful therapeutic modality to reduce risk factors associated with the pathogenesis of IR in obesity.
Similar content being viewed by others
Introduction
Obesity is an independent risk factor for type 2 diabetes mellitus, cardiovascular disease, stroke and cancer.1 Obesity is associated with chronic low-grade systemic inflammation, in part because of increase in proinflammatory systemic milieu imposed by proinflammatory cytokines.2 Adipocytes are a major source of proinflammatory cytokines and are implicated in the onset and progression of obesity, insulin resistance (IR) and cardiovascular disease.2 The proinflammatory adipocytokines, leptin, interleukin-6 (IL-6) and C-reactive protein (CRP), are higher, whereas adiponectin, the main anti-inflammatory and insulin-sensitizing compound, is lower in obesity.3 Adipocytokines modulate various aspects of skeletal muscle metabolism, and therefore any alteration in cytokine secretome during obesity may negatively modulate insulin sensitivity. It is also suggested that increased skeletal muscle oxidative stress may link adiposity and chronic inflammation.4 The mechanisms underlying IR in obesity remain unclear. We believe that the pro-inflammatory stress signals initiated through altered secretion of pro-inflammatory cytokines and oxidative stress form the etiological basis for IR.
Although acute bout of endurance exercise induces a transient oxidant pulse, endurance exercise training results in an increase in skeletal muscle oxidative capacity,5, 6 antioxidant enzymes7 and insulin sensitivity.8 These findings are consistent with the hormesis hypothesis that adaptations induced by repetitive acute exposures to regular exercise-induced oxidant stress lead to long-term metabolic and redox maintenance. Exercise training-mediated redox adaptations occur through activation of signaling pathways that lead to increased synthesis of enzymatic and nonenzymatic antioxidants9, 10 that maintain the ‘physiological’ reactive oxygen species (ROS) levels where they can act as signaling molecules.11
In overweight/obese individuals, the effects of endurance training on oxidative stress and inflammation remain unclear. In lean and obese men, endurance training without weight loss lowers circulating IL-6 and leptin concentrations with no effect on CRP level.12 However, other studies have argued an independent effect of exercise training on inflammatory markers and indicate that weight loss might be required to normalize these levels.13, 14 We have previously reported an improvement in cellular skeletal muscle redox status without alteration in systemic inflammation in obese women subjected to aerobic training that led to higher aerobic capacity, independent of weight loss.15 Few studies directly examined the association between oxidative stress, inflammation and chronic exercise training in adult obese men and the findings remain contradictory.16, 17
We hypothesize that peripheral IR stems from the combination of factors prevalent in obesity, including skeletal muscle oxidative stress and systemic low-grade inflammation, in part because of pro-inflammatory cytokine imbalance. We further hypothesize that endurance exercise training modulates these factors, which results in improvement in IR, in part, by augmenting cellular antioxidant mechanisms. In the current study, we examined the relationship between adiposity, proinflammatory cytokines and oxidative stress levels in a cohort of nondiabetic lean and obese men, group matched for age and physical fitness. We also investigated the therapeutic effects of endurance training on markers of oxidative damage, inflammation and proinflammatory cytokine levels, independent of changes in body composition.
Materials and methods
Subjects
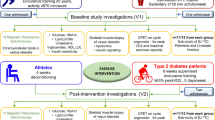
The Research Ethics Board of McMaster University approved the experimental protocol (Research Ethics Board project no. 05–053) that was conducted in accordance with the guidelines of the Declaration of Helsinki. All subjects provided written informed consent before enrollment in the study. Inclusion criteria included age from 20 to 55 years and body mass index (BMI) of ⩾30.0 kg m−2 for obese and 18.5–24.9 kg m−2 for lean individuals with a self-reported stable body weight during the previous 6 months. Exclusion criteria included evidence of diabetes, hypertension (⩾140/90 mm Hg) and/or an abnormal exercise stress test. Individuals who smoked, exercised for >3.5 h per week at a level more vigorous than walking for the preceding 6 months, had orthopedic contraindications to physical activity or used lipid-lowering, glucose-lowering, antihypertensive, antidepressant or weight-loss medications were also excluded. Diet-related exclusion criteria included more than two alcoholic beverages per day. A total of 24 men enrolled in the study, and experimental groups were matched for age and training status (VO2peak per kg FFM per min) when corrected for fat-free mass (FFM) (Table 1). Six men (3 in the lean group and 3 in the obese group) did not complete the study because of inability/unwillingness to comply with protocol or because of personal or work-related conflicts.
Protocol
All subjects underwent a 12-week endurance training protocol on a stationary cycle ergometer (Monarck, Cardio Care 827E, Vansbro, Sweden), as previously described.18 Briefly, the protocol commenced with two 30-min biking sessions at 50% VO2peak per week in the first week and increased to three 60-min biking session at 70% VO2peak per week by the final week of training.
Metabolic assessments
Before and after the intervention, all subjects underwent evaluation of IR, body composition and physical fitness, had a muscle biopsy (vastus lateralis) and completed 3-day diet records (2 weekdays and 1 weekend day). Dietary intake was determined using dietary analysis software (Nutritionist Pro, Version 2.2, First DataBank Inc., San Bruno, CA, USA). Subjects did not exercise for 48 h preceding the metabolic assessments. After an overnight fast, the glycemic response to a 75-g oral glucose load (300 ml) was determined. Blood samples were collected before and 30, 60, 90 and 120 min during the oral glucose tolerance test. For assessment of IR, the homeostasis model assessment index of insulin resistance (HOMA-IR) was determined according to the equation: HOMA-IR=I0 × G0/22.5, where I0 is the fasting insulin concentration (in μU ml−1) and G0 is the fasting glucose concentration (in mM).19 Fat mass, FFM and body fat percentage were assessed by dual-energy X-ray absorptiometry (GE Lunar, Prodigy, Madison, WI, USA). A symptom-limited maximal oxygen consumption test (VO2peak) was determined on an electronically braked cycle ergometer and a computerized open-circuit gas collection system (Moxus Modulator VO2 system with O2 analyzer S-3A/I and CO2 analyzer CD-3A, AEI Technologies Inc., Pittsburgh, PA, USA). Subjects cycled (Excalibur Sport, Lode, Groningen, The Netherlands) at 50 W for 1 min, thereafter increasing in increments of 25 W per min. VO2peak was established when O2 consumption values reached a plateau or was the highest value during the incremental ergometer protocol, pedal revolutions could not be maintained over 60 r.p.m. despite vigorous encouragement and the respiratory exchange ratio was more than 1.12. Subjects were monitored using a 12-lead electrocardiogram to rule out any cardiovascular abnormalities.
Blood analysis
Blood samples were taken from the antecubital vein after an overnight fast and collected in heparinized or untreated vials, placed on ice, centrifuged at 1750 g for 10 min and stored at −80 °C until subsequent analysis. Serum free-fatty acid (FFA) and cytokines were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits: FFA (HR Series NEFA-HR (2); Wako Diagnostics, Richmond, VA, USA); adiponectin (Human Adiponectin Kit DRP300; Quantikine, R&D Systems, Minneapolis, MN, USA), leptin (Human Leptin Kit DLP00, Quantikine, R&D Systems) and IL-6 (Human IL-6 Kit D6050, Quantikine, R&D Systems), and CRP (Human CRP ELISA kit, Alpha Diagnostics International, Cedarlane Labs, Burlington, ON, Canada). Plasma glucose concentration was determined using an automated glucose analyzer (2300 STAT plus, YSI, Hampshire, UK). Plasma insulin concentration was determined using a commercially available ELISA kit (INS-EASIA, Kit KAP1251, BioSource, Nivelles, Belgium).
Urine analysis
Subjects were asked to collect their urine over a 24-h period. Urine samples were stored at −80 °C until subsequent biochemical determination. Urine samples were analyzed for markers of oxidative stress using commercially available ELISA kits: 8-isoprostane (Kit 516351, Cayman Chemical, Ann Arbor, MI, USA) and 8-hydroxy-2-deoxyguanosine (8-OHdG; New 8-OHdG Check, Kit KOG–200S/E, JaICA, Shizuoka, Japan).
Muscle biopsies
Samples of vastus lateralis were obtained by percutaneous suction-modified Bergström needle biopsy, as previously described.20 Biopsies were taken from the same leg before and after the intervention with 3–5 cm between the incision sites. Approximately 120 mg of muscle tissue was obtained each time and immediately dissected of any adipose and connective tissue. Muscle tissue was immediately stored in liquid nitrogen until transferred to −80 °C to be stored for subsequent biochemical and molecular analysis.
Homogenization
Total protein was extracted from frozen skeletal muscle biopsy samples and quantified using the Lowry assay, as previously described.21, 22
Immunoblotting
Proteins were resolved on 7.5, 10 or 12.5% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) gels depending on the molecular weight of the protein of interest. The gels were transferred onto Hybond ECL nitrocellulose membranes (Amersham, Piscataway, NJ, USA) and immunoblotted using the following commercially available primary antibodies: anti-4-hydroxy-2-nonenal (4-HNE; ab48506-50), anti-Cu/ZnSOD (ab16831-100), anti-MnSOD (ab13534-50) and anti-catalase (ab16731), all purchased from Abcam Inc. (Cambridge, MA, USA). Anti-actin (612657, BD Biosciences, Mississauga, ON, Canada) was used as a loading control. Membranes were then incubated with the appropriate anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody and visualized by enhanced chemiluminescence detection reagent (Amersham). Relative intensities of the protein bands were digitally quantified (ImageJ, Version 1.37, NIH, Bethesda, MD, USA).
Protein carbonyl
Total protein carbonyl (PC) content in muscle lysates was determined using a commercially available kit (Oxyblot Protein Oxidation Detection Kit S7150, Chemicon International, Inc., Temecula, CA, USA). Briefly, 10 μl of 12% SDS and 20 μl of 2,4-dinitrophenylhydrazine were added to 10 μl of muscle homogenate and incubated for 15 min at room temperature. Then, 17 μl of neutralizing solution was added to the samples followed by the addition of 6 μl β-mercaptoethanol (1:1.7 dilution with double-distilled H2O). Proteins were resolved on 12.5% SDS-PAGE and transferred onto a Hybond ECL nitrocellulose membrane (Amersham). Relative intensities of the protein bands were digitally quantified (ImageJ, Version 1.37, NIH).
Statistical analysis
When analyzing differences between lean and obese individuals, statistical analyses were completed using unpaired Student’s t-tests for independent samples (Statistica, Version 5.0, Statsoft, Tulsa, OK, USA) with adiposity (lean, obese) being the experimental condition. A two-way repeated measures analysis of variance (Statistica, Version 5.0, Statsoft) with adiposity (lean, obese) and training (pre, post) being the experimental conditions was completed when analyzing the effect of the endurance exercise program. When statistical significance was achieved, Tukey’s HSD (Honestly Significant Difference) post hoc test was used to identify individual differences. Correlation analyses were performed using GraphPad Prism (Version 4, GraphPad Software, San Diego, CA, USA). We used a one-tailed test with urinary markers of oxidative damage (8-OHdG and 8-isoprostane), skeletal muscle PCs and 4-HNE content, as well as plasma adipokines (leptin, adiponectin) and markers of inflammation (CRP, IL-6) because we a priori hypothesized that obese individuals would have higher concentrations of these markers (8-OHdG, 8-isoprostane, PC, 4-HNE, CRP, IL-6 and leptin), but lower adiponectin levels compared with the lean group; and would be influenced by exercise, based upon earlier work by our group.15 For all other analyses, a two-tailed test was employed. Statistical significance was established at P⩽0.05. Data are presented as means±s.e.m.
Results
Subject characteristic
Before training, obese men had significantly higher body weight, BMI, waist circumference, fat mass, FFM, total body fat percentage and android, gynoid, trunk and leg fat percentage than lean men (Table 1). Before training, obese men had significantly higher serum triglyceride and FFA concentrations, but lower serum high-density lipoprotein cholesterol concentrations, than lean men (Table 1). Endurance training significantly increased aerobic capacity by 18% in lean men and by 15% in obese men (P⩽0.001) (Table 1). Endurance training reduced waist circumference by 4% in lean men and by 3% in obese men (P⩽0.001), whereas it had no effect on body weight, BMI, fat mass, FFM, total body fat percentage and android and trunk fat percentage, which remained markedly higher in the obese group (P⩽0.02; Table 1). Endurance training also reduced gynoid and leg fat percentage by −3% and −4%, respectively. Endurance training increased FFA concentrations (P⩽0.03), whereas total cholesterol, triglyceride, high-density lipoprotein and low-density lipoprotein cholesterol concentrations remained unchanged (Table 1). Total energy intake was similar between lean and obese groups before training, and tended to decrease following training with no effect on the composition of the diet (Table 2). Obese men tended to obtain a greater portion of their daily energy intake from fat and less from carbohydrate compared with lean men before and following training (Table 2).
Oral glucose tolerance test and IR
Before training, obese men had significantly higher plasma insulin area under the curve (AUCinsulin, Figure 1a) and higher plasma AUCglucose (Figure 1b) than lean men after the oral glucose challenge. Before training, fasting and 2-h plasma insulin levels as well as 2-h plasma glucose levels were significantly higher in obese men than in lean men (Table 1). Endurance training reduced both the 2-h plasma glucose (P⩽0.01) and insulin (P=0.07) concentrations (Table 1). However, post-training plasma AUCinsulin concentrations remained significantly higher in obese vs lean men (Figure 1a). There was no difference in the plasma AUCglucose concentrations between obese and lean men following training (Figure 1b). Before training, HOMA-IR was 68% higher in obese than in lean men (3.31±0.47 vs 1.97±0.26; P⩽0.02; Figure 1c). Endurance training tended to decrease HOMA-IR by 17% (2.17±0.19 vs 2.60±0.30, post- vs pre-training, respectively; P=0.10). Notably, post-training HOMA-IR values were not different between the two groups (2.51±0.27 vs 1.87±0.25, obese vs lean, respectively; Figure 1c).
Results of the oral glucose tolerance test (OGTT). Mean plasma concentrations of insulin (a) and glucose (b) during a 75-g OGTT, and (c) box and whisker plot, as medians and interquartile range, of the HOMA-IR in lean (n=9) and obese (n=9) men before and following 12 weeks of endurance training. (a) Obese men had significantly higher OGTT insulin area under the curve (AUCinsulin) than lean men before training (P=0.02) and after training (P=0.02). *Pretraining fasting plasma insulin concentration, obese vs. lean P⩽0.03; †Pretraining 2-h plasma insulin concentration, obese vs lean P⩽0.02; ‡Post- vs pre-training 2-h plasma insulin concentration P=0.07. (b) Obese men had higher OGTT AUCglucose than lean men before training (P=0.04). *Pretraining 2-h plasma glucose concentration, obese vs lean P⩽0.01; †Post- vs pre-training 2-h plasma glucose concentration P⩽0.01. (c) Before training, obese men had 68% higher HOMA-IR than lean men. Endurance training reduced HOMA-IR by 5% in the lean men and by 24% in the obese men (P=0.10 vs pretraining). *Pretraining, obese vs lean P⩽0.02. Data are presented as means±s.e.m.
Inflammation and adipocytokines
Before training, serum adiponectin concentration (−27%, 6198±507 vs 8505±473 ng ml−1, respectively; P⩽0.01) and the adiponectin-to-leptin ratio (−81%, 570±126 vs 2976±542, respectively; P⩽0.001) were significantly lower (Table 3), whereas CRP (+737%, 2611±353 vs 312±84 ng ml−1, respectively; P⩽0.0001), IL-6 (+85%, 0.97±0.20 vs 0.52±0.08 pg ml−1, respectively; P⩽0.03) and leptin (+262%, 12 966±1787 vs 3583±543 pg ml−1, respectively; P⩽0.0001) concentrations were markedly higher in obese than in lean men (Figure 2). Endurance training significantly reduced serum leptin concentrations (−33%, 5524±919 vs 8274±1454 pg ml−1, post- and pre-training, respectively; P⩽0.01), with a greater reduction observed in obese men (−34%) than in lean men (−22%); however, post-training serum leptin remained significantly elevated in obese men (P⩽0.001; Figure 2b). Endurance training tended to reduce serum IL-6 (−21%, 0.58±0.07 vs 0.73±0.11 pg ml−1, post- vs pre-training, respectively; P=0.07; Figure 2c); however, post-training serum IL-6 remained significantly elevated in obese than in lean men (P⩽0.02). Serum adiponectin and CRP concentrations were unaltered by endurance training (Figures 2a and d). The adiponectin-to-leptin ratio was also unaltered by training (Table 3).
Blood markers of inflammation and adipokines. (a) Adiponectin, (b) leptin, (c) IL-6 and (d) CRP in lean (n=9) and obese (n=9) men before and after 12 weeks of endurance training. (a) *Pretraining, obese vs lean P⩽0.01. (b) *Pretraining, obese vs. lean P⩽0.0001; †Post- vs pre-training, P⩽0.01. (c) *Pretraining, obese vs. lean P⩽0.03; post- vs pre-training, P=0.07. (d) *Pretraining, obese vs lean P⩽0.0001. Data are presented as means±s.e.m.
Urinary markers of oxidative damage
Before training, obese men tended to have higher urinary 8-OHdG (a marker of DNA damage) and 8-isoprostane (a marker of lipid peroxidation) levels than in lean men (both P=0.09; Figures 3a and b). Endurance training tended to reduce urinary 8-OHdG levels by 15% in obese men only (P=0.07). Endurance training reduced urinary 8-isoprostane levels by 26% (P⩽0.02). Examining the groups independently, the post-training reduction in 8-isoprostane levels was clearly because of the reduction evident only in obese men (−36%; P⩽0.02). Post-training, urinary 8-OHdG and 8-isoprostane levels were not different between obese and lean men (Figures 3a and b).
Urinary and skeletal muscle markers of oxidative damage. (a) 8-OHdG and (b) 8-isoprostane in 24-h urine samples, (c) PC and (d) 4-HNE protein content assessed by western blot in the vastus lateralis of lean (n=9) and obese (n=9) men before and after 12 weeks of endurance training. (a) Pretraining, obese vs lean P=0.09; *Obese men, post- vs pre-training P=0.07. (b) Pretraining, obese vs lean P=0.09; *Obese men, post- vs pre-training P⩽0.02. (c) *Pretraining, obese vs lean P⩽0.02; †Obese men, post- vs pre-training P⩽0.05. (d) *Pretraining, obese vs lean P⩽0.03; †Post- vs pre-training P⩽0.04. Data are presented as means±s.e.m.
Skeletal muscle oxidative damage and antioxidant capacity
Before training, obese men had higher PC (a marker of protein oxidation) and 4-HNE (a marker of lipid peroxidation) levels by 63% (P⩽0.02) and 37% (P⩽0.03), respectively, than in lean men (Figures 3c and d). Endurance training significantly reduced PC levels by 21% (P⩽0.05). Examining the groups independently, endurance training significantly decreased PC levels by 33% in obese men only (P⩽0.05), thus approaching the levels observed in the lean group. Notably, PC levels were not different between the two groups post-training. Endurance training decreased 4-HNE levels by 10% (P⩽0.04); however, they remained higher in obese than in lean men post-training (P⩽0.04).
Before training, only the cytoplasmic antioxidant enzyme copper/zinc superoxide dismutase (Cu/ZnSOD) was higher in the obese than in lean men by 84% (P⩽0.01; Figure 4a). Endurance training tended to increase Cu/ZnSOD by 27% (P=0.10), with a greater increase observed in obese men than in lean men (+37% vs +12%, obese vs lean, respectively; P=0.08). Endurance training increased the mitochondrial antioxidant enzyme manganese SOD(MnSOD) by 47% (P⩽0.01), with a greater increase observed in obese men (+89% vs +13%, obese vs lean, respectively; P=0.01; Figure 4b). Neither obesity nor endurance training altered the protein content of catalase (Figure 4c).
Skeletal muscle markers of antioxidant capacity. (a) Cu/ZnSOD, (b) MnSOD and (c) catalase protein content assessed by western blot in the vastus lateralis of lean (n=9) and obese (n=9) men before and after 12 weeks of endurance training. (a) *Pretraining, obese vs lean P⩽0.01; Obese men, post- vs pre-training P=0.08. (b) *Post- vs. pre-training P⩽0.01. Data are presented as means±s.e.m.
Correlations
A correlation summary between markers of oxidative damage and inflammation vs anthropometric indices of adiposity and IR is given in Table 4. Briefly, markers of inflammation in blood (CRP, IL-6) correlated positively with anthropometric measures (waist circumference, BMI, body fat percentage); however, only CRP was significantly correlated with HOMA-IR. Serum leptin correlated positively with anthropometric indices and HOMA-IR, whereas serum adiponectin and the adiponectin-to-leptin ratio were inversely correlated with these variables. Both urinary 8-OHdG and 8-isoprostane correlated positively with anthropometric indices and HOMA-IR. Skeletal muscle markers of protein oxidative damage (PCs) and lipid peroxidation (4-HNE), as well as antioxidant capacity (Cu/ZnSOD), correlated positively with anthropometric indices of adiposity. A correlation summary between proinflammatory cytokines and oxidative and antioxidant markers is given in Table 5. In general, proinflammatory cytokines (that is, CRP and IL-6) positively correlated with markers of oxidative stress but not with markers of antioxidant capacity (Table 5). Leptin correlated positively whereas the adiponectin-to-leptin ratio correlated negatively with markers of oxidative stress, but neither was correlated with markers of antioxidant capacity (Table 5).
Discussion
In this study, we assessed the association between adiposity, inflammation and oxidative damage in nondiabetic lean and obese men and the therapeutic effects of endurance exercise to negatively regulate these pathologies. We showed that obese men had elevated skeletal muscle and urinary oxidative stress, and dysregulated cytokine profile compared with age- and fitness-matched lean counterparts. We further report that 3 months of endurance exercise training substantially improved biochemical, oxidative stress, inflammation, metabolic health indices and antioxidant variables in sedentary lean and obese participants, independent of weight loss. These results corroborate our previous findings that exercise training lowered oxidative stress markers of lipid peroxidation and DNA damage in lean and obese women following 12 weeks of exercise training.15 A novel aspect of the current study is that the exercise training mediated improvement of skeletal muscle and systemic markers of oxidative damage and skeletal muscle antioxidant capacity in obese men and occurred in the absence of changes in body weight, BMI or body fat percentage, suggesting that positive cellular metabolic and redox changes precedes weight loss. These data provide molecular support to the anecdotal notion that it is better to be fit and obese than lean and sedentary.
Obesity is strongly associated with higher oxidative stress in skeletal muscle.3 One possible obesity-related contributing factor to IR is oxidative stress-induced lipid peroxidation and the modification of proteins by reactive aldehydes such as 4-HNE, presumably because of an inadequacy of antioxidant defenses in tissues.23 Recently, Finkel and Holbrook24 stated that the best strategy to enhance endogenous antioxidant levels may actually be physiological transient pulses of oxidative stress itself, based on the classical physiological concept of hormesis. Exercise at high intensity causes a pulsatile increase in oxidative stress because of the generation of ROS that initially exceed the defense capacity in skeletal muscle.25, 26 However, it has been consistently observed that individuals undergoing exercise training have high levels of antioxidant enzymes and nonenzymatic antioxidants in muscle and demonstrate lower basal levels of oxidative stress and damage accumulation, as well as greater resistance to exercise-induced oxidative stress.7, 27 These adaptations result from cumulative effects of repeated exercise bouts on gene expression and total content of antioxidant enzymes.
Chronically elevated levels of ROS are an important trigger for IR28 and subsequent pathophysiology.29, 30 Thus, the potential clinical significance of lowering ROS levels in skeletal muscle through regular physical activity may ameliorate skeletal muscle IR. In the present study, we showed that obese men have elevated skeletal muscle 4-HNE levels compared with healthy lean subjects and that 4-HNE levels correlate well with anthropometric indices of adiposity, namely waist circumference, BMI and body fat percentage. These findings align with the accumulating evidence suggesting that 4-HNE may play an important role in the pathogenic cellular changes that cause IR and other abnormalities in obesity, and that 4-HNE may also mediate disease processes promoted by obesity.3, 31 Notably, we and others have found that levels of 4-HNE are higher in the blood and/or muscle tissue of obese than in lean subjects.15, 32, 33 Regular moderate exercise is widely prescribed as countermeasure for obesity and associated comorbidities.15, 18 Obese subjects23 and a genetic animal model of extreme overeating34 have lower circulating by-products of lipid peroxidation, and improved muscle insulin sensitivity, following exercise training.23, 34, 35 Indeed, the observed lower 4-HNE levels, along with an improvement in glycemic regulation, in obese subjects post training supports the aforementioned observations. Similarly, high PC levels are observed in IR and are correlated with the sequelae of diabetes.36 We also showed elevated skeletal muscle PC levels in obese men compared with normal-weight controls and a strong positive correlation between PCs and several clinical indices of adiposity. Furthermore, skeletal muscle PC levels in the obese group decreased with training, approaching levels observed in controls; findings consistent with previous reports.37
Intracellular enzymes such as SOD and catalase act as primary line of defense to cope with the deleterious effects of ROS,38 thereby contributing to an overall decrease in oxidative damage. Lower SOD and catalase activity are associated with IR,39 suggesting that reduced capacities of antioxidant enzymes lead to increased oxidative stress in diabetes40 and obesity.41 Although delineating the molecular mechanisms mediating stress-induced IR was beyond the scope of the present study, a great body of studies has reported that oxidative stress is linked to IR.42, 43, 44 It has been suggested that the accumulation of modified cellular components such as proteins, lipids or DNA in obesity activate inhibitory signaling cascades (that is, serine phosphorylation of insulin receptor substrates) or the ‘stress-activated protein kinases’ of the mitogen-activated protein kinase family.45 Elevated ROS stimulates serine/threonine phosphorylation of insulin receptor substrate-1, preventing tyrosine phosphorylation and the subsequent insulin signaling cascade leading to IR.46 Houstis et al.28 demonstrated evidence of a causal role of oxidative stress in IR. Using cellular models, they found that increases in ROS levels preceded the onset of detectable IR.28 In addition, they found that PC levels, a marker of cumulative oxidative stress, were elevated by 50% and 110%, respectively.28 Furthermore, they showed that MnTBAP, an antioxidant molecule that has catalytic activities similar to the ROS-scavenging enzymes SOD and catalase, had a dose-dependent suppression of IR and also prevented the increase in protein carbonylation.28 In another study, Furukawa et al.3 demonstrated in several mouse models of obesity that oxidative stress in accumulated fat mediates the obesity-associated development of metabolic syndrome in the following ways: (1) increased oxidative stress in accumulated fat leads to dysregulated production of adipocytokines and (2) the selective increase in ROS production in accumulated fat leads to elevation of systemic oxidative stress. Importantly, their study revealed that treatment with the NADPH oxidase inhibitor apocynin reduced ROS production in adipose tissue of KKAy mice.3 It also improved hyperinsulinemia, hyperglycemia, hypertriglyceridemia and hepatic steatosis.3 Increased expression of adiponectin and decreased expression of tumor necrosis factor-α were observed in white adipose tissue of apocynin-treated KKAy mice, demonstrating that reduction of oxidative stress in accumulated fat could improve the dysregulation of adipocytokines in vivo.3 Similar to our hypothesis, Furukawa et al.3 proposed that that increased oxidative stress is an early instigator and one of the important underlying causes of obesity-associated sequelae; hence, the redox state in obesity is a potentially useful target in new therapies against obesity-associated metabolic syndrome,3 as we demonstrated in the endurance training study.
Interestingly, we found a higher protein content of Cu/ZnSOD in obese than in lean men before exercise, contrary to other studies.41 This increase in Cu/ZnSOD protein content likely reflects a compensatory upregulation in response to chronically elevated ROS production, as reflected in the higher 4-HNE and PC levels in obese men in the current study. We found a significant increase in MnSOD protein expression concomitant with reductions in skeletal muscle 4-HNE, PCs and urinary 8-OHdG and 8-isoprostane content in the obese men after exercise training. Our observation of an increase in MnSOD protein content in response to endurance exercise training is consistent with previous findings,9, 10 and points to an adaptive response to exercise training.47 Collectively, the reductions in urine and muscle of obese and lean men in response to exercise training in (1) lipid peroxidation, (2) protein oxidation and (3) DNA damage, as well as (4) elevated muscle antioxidant capacity, suggest that exercise training leads to a greater ability of muscle to readily and rapidly detoxify ROS. This is compatible with the prevention of the formation of pathological levels of ROS that may lead to nonspecific ROS-induced cellular damage and redox imbalance,48 thus explaining why trained individuals display less cell damage than untrained subjects.49
Dysregulated proinflammatory cytokine production and reduced adiponectin levels contribute to the pathogenesis of obesity-associated cardiometabolic risk and/or type 2 diabetes mellitus.50 Abdominal and visceral adiposity correlate with systemic biomarkers of oxidative stress in both men and women,3 and increased oxidative stress in obesity may in part be the underlying cause of systemic low-grade chronic inflammation and the development of IR.3 Here we have demonstrated that in nondiabetic obese and lean men, clinical indices of adiposity closely correlated with systemic biomarkers of oxidative DNA and lipid damage, 8-OHdG and 8-isoprostane, respectively. This is in agreement with recent studies suggesting that systemic oxidative stress correlates with BMI and waist circumference.3 In addition, we demonstrated that plasma proinflammatory cytokines and adiponectin levels in obese men were dysregulated compared with healthy lean controls and that oxidative stress correlated positively with CRP and leptin, and inversely with adiponectin. These results are consistent with a recent study that demonstrated that increased ROS in obesity caused dysregulation of proinflammatory cytokine production and that treatment with antioxidants in vivo mitigates this dysregulation, resulting in attenuation of glucose intolerance.3 Indeed, plasma adiponectin levels were markedly lower and correlated inversely with both oxidative stress and IR, whereas leptin, IL-6 and CRP were elevated in the obese group, with leptin and CRP levels correlating positively with both oxidative stress and IR. Several studies have also found an effect of obesity on plasma adiponectin, leptin, IL-6 and CRP.51 Furthermore, exercise training mitigated some of the proinflammatory cytokine dysregulation, namely lowering leptin and IL-6, concomitant with significant reductions in systemic biomarkers of oxidative stress and glucose/insulin in obese men only. Although we found that exercise training reduced oxidative stress in obese and lean men independent of weight loss, there was no effect of training on adiponectin and CRP in either group. This finding is consistent with numerous studies showing that weight loss is needed to increase adiponectin and to normalize certain inflammatory markers.14
In addition, during recent years, accumulating data have shown that muscle cells are able to produce and secrete cytokines and other peptides in response to exercise52, 53 that may exert autocrine, paracrine or endocrine effects. Hence, the source of the elevated proinflammatory cytokine profile cannot be conclusively confirmed as adipocyte derived, nor the reduction in these molecules post training be attributed to a reduction in adipocyte-derived adipokines in the current study. Exercise may reduce macrophage infiltration into adipose tissue in order to reduce inflammation.54 Although the source of the proinflammatory cytokines and oxidative stress markers remains unknown in the current study or almost any human studies conducted to date, we speculate that these results are likely of muscle origin because of the absence of weight loss and body fat percentage. However, future work is indeed warranted to identify if tissues other than adipose tissue (that is, skeletal muscle), which are known to secrete myokines, have an additive effect to the proinflammatory milieu that we see in the current obese population. The finding that muscles produce and release myokines provides a conceptual basis for understanding some of the molecular mechanisms underlying organ cross-talk, including muscle–liver and muscle–fat cross-talk; however, delineating this is beyond the scope of the current study.
The observed reduction in waist circumference in the absence of total body weight loss or reduction in total percent body fat with endurance exercise training is hypothesized to be because of a loss in visceral adipose tissue or trunk fat. To test this hypothesis, we examined region body fat percentages (android, gynoid, trunk and leg fat percentage) assessed by dual-energy X-ray absorptiometry. Counter to our hypothesis, the reduction in waist circumference could not be explained by android or trunk fat percentage. It is not easy to reconcile these findings, but it can be speculated that visceral fat and/or trunk fat depots may have been mobilized and redistributed to other regions not sensitive enough to be captured by dual-energy X-ray absorptiometry scan or assessed in the present study. Furthermore, a single, skilled clinical study coordinator experienced in performing multiple physical anthropometric measurements in humans made both pre- and post-training assessments; hence we are confident in our measurements. In addition, a similar observation was made by our group in a cohort of obese women who underwent a similar exercise training regimen.15
In the present study, we evaluated markers of oxidative damage and inflammation to ascertain a broader perspective regarding redox balance and fitness level while simultaneously controlling for age, physical fitness, weight loss and body composition. When lean and obese subjects completed 3 months of endurance exercise training, their levels of systemic and skeletal muscle oxidative stress decreased and their circulating marker of inflammation had a strong decreasing trend. In addition, multiple glucose homeostatic health indicators improved concomitant with a strong decreasing trend in IR. We propose that endurance exercise is a therapeutic modality that reduces risk factors associated with the pathogenesis of IR in obesity.
References
Hubert HB, Feinleib M, McNamara PM, Castelli WP . Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983; 67: 968–977.
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191–198.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004; 114: 1752–1761.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr . Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808.
Holloszy JO . Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 1967; 242: 2278–2282.
Holloszy JO, Booth FW . Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 1976; 38: 273–291.
Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA . Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 2005; 19: 1498–1500.
Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Kashyap S et al. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism 2004; 53: 1233–1242.
Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL . Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol 1994; 267: R439–R445.
Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL . Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol 1997; 272: R363–R369.
Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S et al. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol 2001; 84: 1–6.
Dekker MJ, Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R et al. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism 2007; 56: 332–338.
Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 2001; 86: 3815–3819.
Yokoyama H, Emoto M, Araki T, Fujiwara S, Motoyama K, Morioka T et al. Effect of aerobic exercise on plasma adiponectin levels and insulin resistance in type 2 diabetes. Diabetes Care 2004; 27: 1756–1758.
Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA . Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med 2008; 45: 503–511.
Pihl E, Zilmer K, Kullisaar T, Kairane C, Magi A, Zilmer M . Atherogenic inflammatory and oxidative stress markers in relation to overweight values in male former athletes. Int J Obes (Lond) 2006; 30: 141–146.
Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol 2006; 100: 1657–1665.
Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA . Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring) 2008; 16: 2281–2288.
Emoto M, Nishizawa Y, Maekawa K, Hiura Y, Kanda H, Kawagishi T et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care 1999; 22: 818–822.
Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA . Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics 2008; 32: 219–228.
Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci USA 2011; 108: 4135–4140.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ . Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–275.
Vincent HK, Innes KE, Vincent KR . Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab 2007; 9: 813–839.
Finkel T, Holbrook NJ . Oxidants, oxidative stress and the biology of ageing. Nature 2000; 408: 239–247.
McArdle A, Jackson MJ . Exercise, oxidative stress and ageing. J Anat 2000; 197 (Pt 4): 539–541.
McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ . Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 2001; 280: C621–C627.
Parise G, Phillips SM, Kaczor JJ, Tarnopolsky MA . Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic Biol Med 2005; 39: 289–295.
Houstis N, Rosen E, Lander E . Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006; 440: 944–948.
Zarkovic N . 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med 2003; 24: 281–291.
Chen ZH, Niki E . 4-hydroxynonenal (4-HNE) has been widely accepted as an inducer of oxidative stress. Is this the whole truth about it or can 4-HNE also exert protective effects? IUBMB Life 2006; 58: 372–373.
Mattson MP . Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol 2009; 44: 625–633.
Russell A, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Deriaz O et al. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Lett 2003; 551: 104–106.
Vincent HK, Powers SK, Dirks AJ, Scarpace PJ . Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord 2001; 25: 378–388.
Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW . Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 2008; 104: 708–715.
Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 2007; 42: 665–674.
Dominguez C, Ruiz E, Gussinye M, Carrascosa A . Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 1998; 21: 1736–1742.
da Silva LA, Pinho CA, Rocha LG, Tuon T, Silveira PC, Pinho RA . Effect of different models of physical exercise on oxidative stress markers in mouse liver. Appl Physiol Nutr Metab 2009; 34: 60–65.
Arrick BA, Nathan CF . Glutathione metabolism as a determinant of therapeutic efficacy: a review. Cancer Res 1984; 44: 4224–4232.
Baynes JW, Thorpe SR . Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 1999; 48: 1–9.
Arai K, Iizuka S, Tada Y, Oikawa K, Taniguchi N . Increase in the glucosylated form of erythrocyte Cu-Zn-superoxide dismutase in diabetes and close association of the nonenzymatic glucosylation with the enzyme activity. Biochim Biophys Acta 1987; 924: 292–296.
Salazar DE, Sorge CL, Jordan SW, Corcoran GB . Obesity decreases hepatic glutathione concentrations and markedly potentiates allyl alcohol-induced periportal necrosis in the overfed rat. Int J Obes Relat Metab Disord 1994; 18: 25–33.
Evans JL, Goldfine ID, Maddux BA, Grodsky GM . Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 2003; 52: 1–8.
Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J . Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett 1995; 368: 225–229.
Hirashima O, Kawano H, Motoyama T, Hirai N, Ohgushi M, Kugiyama K et al. Improvement of endothelial function and insulin sensitivity with vitamin C in patients with coronary spastic angina: possible role of reactive oxygen species. J Am Coll Cardiol 2000; 35: 1860–1866.
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A . Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev 2009; 89: 27–71.
Bloch-Damti A, Bashan N . Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal 2005; 7: 1553–1567.
Lu D, Maulik N, Moraru II, Kreutzer DL, Das DK . Molecular adaptation of vascular endothelial cells to oxidative stress. Am J Physiol 1993; 264: C715–C722.
Gomez-Cabrera MC, Domenech E, Vina J . Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 2008; 44: 126–131.
Radak Z, Apor P, Pucsok J, Berkes I, Ogonovszky H, Pavlik G et al. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci 2003; 72: 1627–1633.
Leiter LA, Fitchett DH, Gilbert RE, Gupta M, Mancini GB, McFarlane PA et al. Cardiometabolic risk in Canada: a detailed analysis and position paper by the cardiometabolic risk working group. Can J Cardiol 2011; 27: e1–e33.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257: 79–83.
Pedersen BK, Febbraio MA . Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8: 457–465.
Pillon NJ, Bilan PJ, Fink LN, Klip A . Cross-talk between skeletal muscle and immune cells: muscle-derived mediators and metabolic implications. Am J Physiol Endocrinol Metab 2013; 304: E453–E465.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA . The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 2011; 11: 607–615.
Acknowledgements
This study was funded by a grant to MAT from the Canadian Institutes of Health Research (CIHR). We sincerely thank the Lammert and Corkins family for their generous donation to the McMaster University Neuromuscular and Neurometabolic Centre. We greatly appreciate Jose Santana for his help with subject testing and supervision of exercise sessions. IAS was supported by the Heart and Stroke Foundation of Ontario Master’s Studentship Award, Ontario Graduate Scholarship (OGS) and the OGS in Science and Technology (OGSST). AS was supported by the CIHR Banting Fellowship, American Federation for Aging Research/The Ellison Medical Foundation Postdoctoral Fellowship, CIHR Institute of Aging Doctoral Award and Canada PRESTIGE Scholarship. IAS, MJH and MAT conceived and designed the study; IAS and MJH were involved in conducting and coordinating the study; IAS and AS researched and analyzed data; IAS wrote the article; IAS, AS, MJH, SR and MAT reviewed and edited the article. All authors read and approved the final article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Samjoo, I., Safdar, A., Hamadeh, M. et al. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr & Diabetes 3, e88 (2013). https://doi.org/10.1038/nutd.2013.30
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2013.30
Keywords
This article is cited by
-
Biochemical changes in oxidative stress markers following endurance training and consumption of purslane seed in rats with hydrogen peroxide-induced toxicity
Sport Sciences for Health (2019)
-
Chronic hepatitis B virus infection and risk of chronic kidney disease: a population-based prospective cohort study of 0.5 million Chinese adults
BMC Medicine (2018)
-
The role of mitochondrial DNA damage at skeletal muscle oxidative stress on the development of type 2 diabetes
Molecular and Cellular Biochemistry (2018)
-
Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression
Scientific Reports (2016)
-
The potential of endurance exercise-derived exosomes to treat metabolic diseases
Nature Reviews Endocrinology (2016)