Abstract
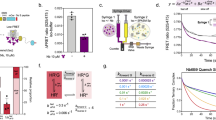
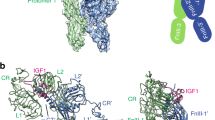
Growth hormone is believed to activate the growth hormone receptor (GHR) by dimerizing two identical receptor subunits, leading to activation of JAK2 kinase associated with the cytoplasmic domain. However, we have reported previously that dimerization alone is insufficient to activate full-length GHR. By comparing the crystal structure of the liganded and unliganded human GHR extracellular domain, we show here that there is no substantial change in its conformation on ligand binding. However, the receptor can be activated by rotation without ligand by inserting a defined number of alanine residues within the transmembrane domain. Fluorescence resonance energy transfer (FRET), bioluminescence resonance energy transfer (BRET) and coimmunoprecipitation studies suggest that receptor subunits undergo specific transmembrane interactions independent of hormone binding. We propose an activation mechanism involving a relative rotation of subunits within a dimeric receptor as a result of asymmetric placement of the receptor-binding sites on the ligand.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fuh, G. et al. Rational design of potent antagonists to the human growth hormone receptor. Science 256, 1677–1680 (1992).
Rowlinson, S.W. et al. Activation of chimeric and full-length growth hormone receptors by growth hormone receptor monoclonal antibodies. A specific conformational change may be required for full-length receptor signaling. J. Biol. Chem. 273, 5307–5314 (1998).
Jiang, J. et al. A conformationally sensitive GHR [growth hormone (GH) receptor] antibody: impact on GH signaling and GHR proteolysis. Mol. Endocrinol. 18, 2981–2996 (2004).
Wan, Y., McDevitt, A., Shen, B., Smythe, M.L. & Waters, M.J. Increased site 1 affinity improves biopotency of porcine growth hormone. Evidence against diffusion dependent receptor dimerization. J. Biol. Chem. 279, 44775–44784 (2004).
Ross, R.J. et al. Binding and functional studies with the growth hormone receptor antagonist, B2036-PEG (pegvisomant), reveal effects of pegylation and evidence that it binds to a receptor dimer. J. Clin. Endocrinol. Metab. 86, 1716–1723 (2001).
Harding, P.A. et al. Growth hormone (GH) and a GH antagonist promote GH receptor dimerization and internalization. J. Biol. Chem. 271, 6708–6712 (1996).
Constantinescu, S.N., Huang, L.J., Nam, H. & Lodish, H.F. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol. Cell 7, 377–385 (2001).
Livnah, O. et al. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 283, 987–990 (1999).
Seubert, N. et al. Active and inactive orientations of the transmembrane and cytosolic domains of the erythropoietin receptor dimer. Mol. Cell 12, 1239–1250 (2003).
McKinstry, W.J. et al. Crystallization and preliminary X-ray diffraction analysis of the unliganded human growth hormone receptor. Acta Crystallogr. D Biol. Crystallogr. 60, 2380–2382 (2004).
de Vos, A.M., Ultsch, M. & Kossiakoff, A.A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255, 306–312 (1992).
Clackson, T., Ultsch, M.H., Wells, J.A. & de Vos, A.M. Structural and functional analysis of the 1:1 growth hormone:receptor complex reveals the molecular basis for receptor affinity. J. Mol. Biol. 277, 1111–1128 (1998).
Ottemann, K.M., Xiao, W., Shin, Y.K. & Koshland, D.E., Jr. A piston model for transmembrane signaling of the aspartate receptor. Science 285, 1751–1754 (1999).
Palczewski, K. et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 (2000).
Rost, B., Yachdav, G. & Liu, J. The PredictProtein server. Nucleic Acids Res. 32, W321–W326 (2004).
Gent, J., van Kerkhof, P., Roza, M., Bu, G. & Strous, G.J. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc. Natl. Acad. Sci. USA 99, 9858–9863 (2002).
Baumgartner, J.W., Wells, C.A., Chen, C.M. & Waters, M.J. The role of the WSXWS equivalent motif in growth hormone receptor function. J. Biol. Chem. 269, 29094–29101 (1994).
Dinerstein, H. et al. The proline-rich region of the GH receptor is essential for JAK2 phosphorylation, activation of cell proliferation, and gene transcription. Mol. Endocrinol. 9, 1701–1707 (1995).
Eidne, K.A., Kroeger, K.M. & Hanyaloglu, A.C. Applications of novel resonance energy transfer techniques to study dynamic hormone receptor interactions in living cells. Trends Endocrinol. Metab. 13, 415–421 (2002).
Damjanovich, S. et al. Preassembly of interleukin 2 (IL-2) receptor subunits on resting Kit 225 K6 T cells and their modulation by IL-2, IL-7, and IL-15: a fluorescence resonance energy transfer study. Proc. Natl. Acad. Sci. USA 94, 13134–13139 (1997).
Couturier, C. & Jockers, R. Activation of the leptin receptor by a ligand-induced conformational change of constitutive receptor dimers. J. Biol. Chem. 278, 26604–26611 (2003).
Issad, T., Boute, N. & Pernet, K. A homogenous assay to monitor the activity of the insulin receptor using Bioluminescence Resonance Energy Transfer. Biochem. Pharmacol. 64, 813–817 (2002).
Waters, M.J. & Friesen, H.G. Purification and partial characterization of a nonprimate growth hormone receptor. J. Biol. Chem. 254, 6815–6825 (1979).
Gurezka, R., Laage, R., Brosig, B. & Langosch, D. A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J. Biol. Chem. 274, 9265–9270 (1999).
Hanyaloglu, A.C., Seeber, R.M., Kohout, T.A., Lefkowitz, R.J. & Eidne, K.A. Homo- and hetero-oligomerization of thyrotropin-releasing hormone (TRH) receptor subtypes. Differential regulation of beta-arrestins 1 and 2. J. Biol. Chem. 277, 50422–50430 (2002).
Kubatzky, K.F. et al. Self assembly of the transmembrane domain promotes signal transduction through the erythropoietin receptor. Curr. Biol. 11, 110–115 (2001).
Bernat, B., Pal, G., Sun, M. & Kossiakoff, A.A. Determination of the energetics governing the regulatory step in growth hormone-induced receptor homodimerization. Proc. Natl. Acad. Sci. USA 100, 952–957 (2003).
Zhang, Y., Jiang, J., Kopchick, J.J. & Frank, S.J. Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J. Biol. Chem. 274, 33072–33084 (1999).
van Kerkhof, P., Smeets, M. & Strous, G.J. The ubiquitin-proteasome pathway regulates the availability of the GH receptor. Endocrinology 143, 1243–1252 (2002).
Schneider, H. et al. Homodimerization of erythropoietin receptor by a bivalent monoclonal antibody triggers cell proliferation and differentiation of erythroid precursors. Blood 89, 473–482 (1997).
Waters, M. The growth hormone receptor. in Handbook of Physiology—Section 7: The Endocrine System., Vol. 5 (ed. Goodman, M.) 445–480 (Oxford University Press, New York, 1999).
Chen, C., Brinkworth, R. & Waters, M.J. The role of receptor dimerization domain residues in growth hormone signaling. J. Biol. Chem. 272, 5133–5140 (1997).
Gent, J., Van Den Eijnden, M., Van Kerkhof, P. & Strous, G.J. Dimerization and signal transduction of the growth hormone receptor. Mol. Endocrinol. 17, 967–975 (2003).
Blott, S. et al. Molecular dissection of a quantitative trait locus: a phenylalanine-to-tyrosine substitution in the transmembrane domain of the bovine growth hormone receptor is associated with a major effect on milk yield and composition. Genetics 163, 253–266 (2003).
Angers, S., Salahpour, A. & Bouvier, M. Biochemical and biophysical demonstration of GPCR oligomerization in mammalian cells. Life Sci. 68, 2243–2250 (2001).
Kroeger, K.M., Hanyaloglu, A.C., Seeber, R.M., Miles, L.E. & Eidne, K.A. Constitutive and agonist-dependent homo-oligomerization of the thyrotropin-releasing hormone receptor. Detection in living cells using bioluminescence resonance energy transfer. J. Biol. Chem. 276, 12736–12743 (2001).
Piper, R.C., Hess, L.J. & James, D.E. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am. J. Physiol. 260, C570–C580 (1991).
Acknowledgements
This work was supported by National Health and Medical Research Council (NHMRC; Australia) project grants to M.J.W. and K.A.E. We thank H. Tong and other staff at BioCARS for their help at the Advanced Photon Source (supported by the US Department of Energy, Basic Energy Sciences, Office of Energy Research). The crystallographic work, and use of the BioCARS sector, was supported by the Australian Synchrotron Research Program, funded by the Commonwealth of Australia under the Major National Research Facilities Program. We thank E. Holliday and E. Lim for their skilled assistance. W.J.M. is a NHMRC Industry Fellow and M.W.P. is an NHMRC Senior Principal Research Fellow and M.J.W. and K.A.E. are NHMRC Principal Research Fellows.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Brown, R., Adams, J., Pelekanos, R. et al. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol 12, 814–821 (2005). https://doi.org/10.1038/nsmb977
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb977
This article is cited by
-
Orchestration of signaling by structural disorder in class 1 cytokine receptors
Cell Communication and Signaling (2020)
-
A dual role for the N-terminal domain of the IL-3 receptor in cell signalling
Nature Communications (2018)
-
Ligand-induced type II interleukin-4 receptor dimers are sustained by rapid re-association within plasma membrane microcompartments
Nature Communications (2017)
-
Pegvisomant: a growth hormone receptor antagonist used in the treatment of acromegaly
Pituitary (2017)
-
Diffusion of Single-Pass Transmembrane Receptors: From the Plasma Membrane into Giant Liposomes
The Journal of Membrane Biology (2017)