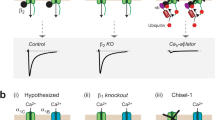
Abstract
Voltage-gated Ca2+ channel β (Cavβ) subunits have a highly conserved core consisting of interacting Src homology 3 and guanylate kinase domains, and are postulated to exert their effects through AID, the major interaction site in the pore-forming α1 subunit. This stereotypical interaction does not explain how individual Cavβ subunits modulate α1 subunits differentially. Here we show that AID is neither necessary nor sufficient for critical Cavβ regulatory properties. Complete modulation depends on additional contacts that are exclusive of AID and not revealed in recent crystal structures. These data offer a new context for understanding Cavβ modulation, suggesting that the AID interaction orients the Cavβ core so as to permit additional isoform-specific Cavα1-Cavβ interactions that underlie the particular regulation seen with each Cavα1-Cavβ pair, rather than as the main site of regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 (2000).
Dolphin, A.C. β subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 35, 599–620 (2003).
Pragnell, M. et al. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature 368, 67–70 (1994).
Chen, Y.H. et al. Structural basis of the α1-β subunit interaction of voltage-gated Ca2+ channels. Nature 429, 675–680 (2004).
Opatowsky, Y., Chen, C.C., Campbell, K.P. & Hirsch, J.A. Structural analysis of the voltage-dependent calcium channel β subunit functional core and its complex with the α1 interaction domain. Neuron 42, 387–399 (2004).
Van Petegem, F., Clark, K.A., Chatelain, F.C. & Minor, D.L. Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature 429, 671–675 (2004).
McGee, A.W. et al. Calcium channel function regulated by the SH3-GK module in β subunits. Neuron 42, 89–99 (2004).
Takahashi, S.X., Miriyala, J. & Colecraft, H.M. Membrane-associated guanylate kinase-like properties of β-subunits required for modulation of voltage-dependent Ca2+ channels. Proc. Natl. Acad. Sci. USA 101, 7193–7198 (2004).
Gao, T., Chien, A.J. & Hosey, M.M. Complexes of the α 1C and β subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. J. Biol. Chem. 274, 2137–2144 (1999).
Opatowsky, Y., Chomsky-Hecht, O., Kang, M.-G., Campbell, K.P. & Hirsch, J.A. The voltage-dependent calcium channel β subunit contains two stable interacting domains. J. Biol. Chem. 278, 52323–52332 (2003).
Bichet, D. et al. The I-II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron 25, 177–190 (2000).
Paarmann, I., Spangenberg, O., Lavie, A. & Konrad, M. Formation of complexes between Ca2+-calmodulin and the synapse-associated protein sap97 requires the SH3 domain-guanylate kinase domain-connecting hook region. J. Biol. Chem. 277, 40832–40838 (2002).
Masuko, N. et al. Interaction of NE-dlg/SAP102, a neuronal and endocrine tissue-specific membrane-associated guanylate kinase protein, with calmodulin and PSD-95/SAP90. A possible regulatory role in molecular clustering at synaptic sites. J. Biol. Chem. 274, 5782–5790 (1999).
Pitt, G.S. et al. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J. Biol. Chem. 276, 30794–30802. (2001).
Restituito, S. et al. The β2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J. Neurosci. 20, 9046–9052 (2000).
Walker, D., Bichet, D., Campbell, K.P. & De Waard, M. A β4 isoform-specific interaction site in the carboxyl-terminal region of the voltage-dependent Ca2+ channel α1a subunit. J. Biol. Chem. 273, 2361–2367 (1998).
Geib, S. et al. The interaction between the I-II loop and the III-IV loop of Cav2.1 contributes to voltage-dependent inactivation in a β-dependent manner. J. Biol. Chem. 277, 10003–10013 (2002).
Sheng, M. & Sala, C. PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 24, 1–29 (2001).
Brenman, J.E. et al. Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J. Neurosci. 18, 8805–8813 (1998).
Naisbitt, S. et al. Characterization of guanylate kinase-associated protein, a postsynaptic density protein at excitatory synapses that interacts directly with postsynaptic density-95/synapse-associated protein 90. J. Neurosci. 17, 5687–5696 (1997).
Deguchi, M. et al. BEGAIN (brain-enriched guanylate kinase-associated protein), a novel neuronal PSD-95/SAP90-binding protein. J. Biol. Chem. 273, 26269–26272 (1998).
Ghosh, S. & Lowenstein, J.M. A multifunctional vector system for heterologous expression of proteins in Escherichia coli. Expression of native and hexahistidyl fusion proteins, rapid purification of the fusion proteins, and removal of fusion peptide by KEX2 protease. Gene 176, 249–255 (1996).
Acknowledgements
This work was supported by the US National Institutes of Health and the Irma T. Hirschl Trust (G.S.P.). We thank J. Riley and S. Siegelbaum for supplying Xenopus oocytes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Maltez, J., Nunziato, D., Kim, J. et al. Essential Cavβ modulatory properties are AID-independent. Nat Struct Mol Biol 12, 372–377 (2005). https://doi.org/10.1038/nsmb909
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb909
This article is cited by
-
Cavβ surface charged residues contribute to the regulation of neuronal calcium channels
Molecular Brain (2022)
-
Increase of CaV3 channel activity induced by HVA β1b-subunit is not mediated by a physical interaction
BMC Research Notes (2018)
-
Functional assessment of three Rem residues identified as critical for interactions with Ca2+ channel β subunits
Pflügers Archiv - European Journal of Physiology (2015)
-
Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond
Nature Reviews Neuroscience (2012)
-
Trafficking and stability of voltage-gated calcium channels
Cellular and Molecular Life Sciences (2012)