Abstract
The prokaryotic tubulin homolog FtsZ polymerizes into a ring structure essential for bacterial cell division. We have used refolded FtsZ to crystallize a tubulin-like protofilament. The N- and C-terminal domains of two consecutive subunits in the filament assemble to form the GTPase site, with the C-terminal domain providing water-polarizing residues. A domain-swapped structure of FtsZ and biochemical data on purified N- and C-terminal domains show that they are independent. This leads to a model of how FtsZ and tubulin polymerization evolved by fusing two domains. In polymerized tubulin, the nucleotide-binding pocket is occluded, which leads to nucleotide exchange being the rate-limiting step and to dynamic instability. In our FtsZ filament structure the nucleotide is exchangeable, explaining why, in this filament, nucleotide hydrolysis is the rate-limiting step during FtsZ polymerization. Furthermore, crystal structures of FtsZ in different nucleotide states reveal notably few differences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Errington, J., Daniel, R.A. & Scheffers, D.J. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67, 52–65 (2003).
Lutkenhaus, J. & Addinall, S.G. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66, 93–116 (1997).
Rothfield, L., Justice, S. & Garcia-Lara, J. Bacterial cell division. Annu. Rev. Genet. 33, 423–448 (1999).
Addinall, S.G. & Holland, B. The tubulin ancestor, FtsZ, draughtsman, designer and driving force for bacterial cytokinesis. J. Mol. Biol. 318, 219–236 (2002).
Erickson, H.P. FtsZ, a prokaryotic homolog of tubulin? Cell 80, 367–370 (1995).
Löwe, J. & Amos, L.A. Crystal structure of the bacterial cell-division protein FtsZ. Nature 391, 203–206 (1998).
Nogales, E., Wolf, S.G. & Downing, K.H. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391, 199–203 (1998).
Nogales, E., Downing, K.H., Amos, L.A. & Löwe, J. Tubulin and FtsZ form a distinct family of GTPases. Nat. Struct. Biol. 5, 451–458 (1998).
Nogales, E., Whittaker, M., Milligan, R.A. & Downing, K.H. High-resolution model of the microtubule. Cell 96, 79–88 (1999).
Oliva, M.A. et al. Assembly of archaeal cell division protein FtsZ and a GTPase-inactive mutant into double-stranded filaments. J. Biol. Chem. 278, 33562–33570 (2003).
Erickson, H.P., Taylor, D.W., Taylor, K.A. & Bramhill, D. Bacterial cell division protein FtsZ assembles into protofilament sheets and minirings, structural homologs of tubulin polymers. Proc. Natl. Acad. Sci. USA 93, 519–523 (1996).
Löwe, J. & Amos, L.A. Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J. 18, 2364–2371 (1999).
Scheffers, D.J., de Wit, J.G., den Blaauwen, T. & Driessen, A.J. Substitution of a conserved aspartate allows cation-induced polymerization of FtsZ. FEBS Lett. 494, 34–37 (2001).
Cordell, S.C., Robinson, E.J. & Löwe, J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc. Natl. Acad. Sci. USA 100, 7889–7894 (2003).
Romberg, L. & Mitchison, T.J. Rate-limiting guanosine 5′-triphosphate hydrolysis during nucleotide turnover by FtsZ, a prokaryotic tubulin homologue involved in bacterial cell division. Biochemistry 43, 282–288 (2004).
Mitchison, T. & Kirschner, M. Dynamic instability of microtubule growth. Nature 312, 237–242 (1984).
Mingorance, J., Rueda, S., Gomez-Puertas, P., Valencia, A. & Vicente, M. Escherichia coli FtsZ polymers contain mostly GTP and have a high nucleotide turnover. Mol. Microbiol. 41, 83–91 (2001).
Huecas, S. & Andreu, J.M. Energetics of the cooperative assembly of cell division protein FtsZ and the nucleotide hydrolysis switch. J. Biol. Chem. 278, 46146–46154 (2003).
Mukherjee, A. & Lutkenhaus, J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J. Bacteriol. 176, 2754–2758 (1994).
Bramhill, D. & Thompson, C.M. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc. Natl. Acad. Sci. USA 91, 5813–5817 (1994).
Ben-Yehuda, S. & Losick, R. Asymmetric cell division in B. subtilis involves a spiral-like intermediate of the cytokinetic protein FtsZ. Cell 109, 257–266 (2002).
Lu, C., Reedy, M. & Erickson, H.P. Straight and curved conformations of FtsZ are regulated by GTP hydrolysis. J. Bacteriol. 182, 164–170 (2000).
Diaz, J.F. et al. Activation of cell division protein FtsZ. Control of switch loop T3 conformation by the nucleotide γ-phosphate. J. Biol. Chem. 276, 17307–17315 (2001).
Andreu, J.M., Oliva, M.A. & Monasterio, O. Reversible unfolding of FtsZ cell division proteins from archaea and bacteria. Comparison with eukaryotic tubulin folding and assembly. J. Biol. Chem. 277, 43262–43270 (2002).
Löwe, J., Li, H., Downing, K.H. & Nogales, E. Refined structure of α β-tubulin at 3.5 Å resolution. J. Mol. Biol. 313, 1045–1057 (2001).
Hyman, A.A., Chretien, D., Arnal, I. & Wade, R.H. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(α,β)-methylene-diphosphonate. J. Cell Biol. 128, 117–125 (1995).
Nogales, E., Whittaker, M., Milligan, R.A. & Downing, K.H. High-resolution model of the microtubule. Cell 96, 79–88 (1999).
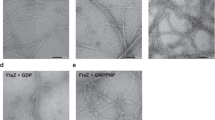
Huecas, S. & Andreu, J.M. Polymerization of nucleotide-free, GDP- and GTP-bound cell division protein FtsZ: GDP makes the difference. FEBS Lett. 569, 43–48 (2004).
Ravelli, R.B. et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428, 198–202 (2004).
Nogales, E., Wang, H.W. & Niederstrasser, H. Tubulin rings: which way do they curve? Curr. Opin. Struct. Biol. 13, 256–261 (2003).
Scheffzek, K. et al. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 (1997).
Daumke, O., Weyand, M., Chakrabarti, P.P., Vetter, I.R. & Wittinghofer, A. The GTPase-activating protein Rap1GAP uses a catalytic asparagine. Nature 429, 197–201 (2004).
Schindelin, H., Kisker, C., Schlessman, J.L., Howard, J.B. & Rees, D.C. Structure of ADP x AIF4(–)-stabilized nitrogenase complex and its implications for signal transduction. Nature 387, 370–376 (1997).
Egea, P.F. et al. Substrate twinning activates the signal recognition particle and its receptor. Nature 427, 215–221 (2004).
Focia, P.J., Shepotinovskaya, I.V., Seidler, J.A. & Freymann, D.M. Heterodimeric GTPase core of the SRP targeting complex. Science 303, 373–377 (2004).
Tesmer, J.J., Berman, D.M., Gilman, A.G. & Sprang, S.R. Structure of RGS4 bound to AlF4-activated Giα1: stabilization of the transition state for GTP hydrolysis. Cell 89, 251–261 (1997).
Stock, D., Perisic, O. & Löwe, J. Nanolitre crystallisation at the MRC Laboratory of Molecular Biology. Prog. Biophys. Mol. Biol. (in the press).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Brunger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Miroux, B. & Walker, J.E. Over-production of proteins in Escherichia coli: Mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289–298 (1996).
Henkel, R.D., van de Berg, J.L. & Walsh, R.A. A microassay for ATPase. Anal. Biochem. 169, 312–318 (1987).
Acknowledgements
We thank the Ministerio de Ciencia y Tecnologia, Spain for financial support to M.A. We also thank J.M. Andreu (Madrid) for supplying us with the MjFtsZ-W319Y plasmid. Finally, we had great support on the following beamlines: Synchtrotron Radiation Source (14.2 and 9.6) and European Synchrotron Radiation Facility (ID29 and BM14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Oliva, M., Cordell, S. & Löwe, J. Structural insights into FtsZ protofilament formation. Nat Struct Mol Biol 11, 1243–1250 (2004). https://doi.org/10.1038/nsmb855
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb855
This article is cited by
-
Structures of a FtsZ single protofilament and a double-helical tube in complex with a monobody
Nature Communications (2023)
-
An Arg/Ala-rich helix in the N-terminal region of M. tuberculosis FtsQ is a potential membrane anchor of the Z-ring
Communications Biology (2023)
-
Mycobacterial FtsZ and inhibitors: a promising target for the anti-tubercular drug development
Molecular Diversity (2023)
-
Halogenation of tyrosine perturbs large-scale protein self-organization
Nature Communications (2022)
-
Treadmilling FtsZ polymers drive the directional movement of sPG-synthesis enzymes via a Brownian ratchet mechanism
Nature Communications (2021)