Abstract
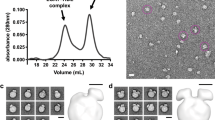
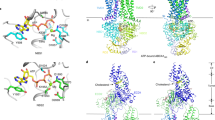
Cholesteryl ester transfer protein (CETP) shuttles various lipids between lipoproteins, resulting in the net transfer of cholesteryl esters from atheroprotective, high-density lipoproteins (HDL) to atherogenic, lower-density species. Inhibition of CETP raises HDL cholesterol and may potentially be used to treat cardiovascular disease. Here we describe the structure of CETP at 2.2-Å resolution, revealing a 60-Å-long tunnel filled with two hydrophobic cholesteryl esters and plugged by an amphiphilic phosphatidylcholine at each end. The two tunnel openings are large enough to allow lipid access, which is aided by a flexible helix and possibly also by a mobile flap. The curvature of the concave surface of CETP matches the radius of curvature of HDL particles, and potential conformational changes may occur to accommodate larger lipoprotein particles. Point mutations blocking the middle of the tunnel abolish lipid-transfer activities, suggesting that neutral lipids pass through this continuous tunnel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
National Center for Chronic Disease Prevention and Health Promotion. Preventing heart disease and stroke. Centers for Disease Control and Prevention http://www.cdc.gov/nccdphp/publications/factsheets/Prevention/cvh.htm (2005).
Gordon, D.J. et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79, 8–15 (1989).
Boden, W.E. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High-Density Lipoprotein Intervention Trial. Am. J. Cardiol. 86, 19L–22L (2000).
Rubins, H.B. et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N. Engl. J. Med. 341, 410–418 (1999).
Shepherd, J., Betteridge, J., Van Gaal, L. & European Consensus Panel. Nicotinic acid in the management of dyslipidaemia associated with diabetes and metabolic syndrome: a position paper developed by a European Consensus Panel. Curr. Med. Res. Opin. 21, 665–682 (2005).
Okamoto, H. et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 406, 203–207 (2000).
Clark, R.W. et al. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler. Thromb. Vasc. Biol. 24, 490–497 (2004).
Brousseau, M.E. et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350, 1505–1515 (2004).
Linsel-Nitschke, P. & Tall, A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4, 193–205 (2005).
Tall, A.R. Plasma cholesteryl ester transfer protein. J. Lipid Res. 34, 1255–1274 (1993).
Barter, P.J. et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 160–167 (2003).
Kawano, K., Qin, S.C., Lin, M., Tall, A.R. & Jiang, X.C. Cholesteryl ester transfer protein and phospholipid transfer protein have nonoverlapping functions in vivo. J. Biol. Chem. 275, 29477–29481 (2000).
Klerkx, A.H. et al. Cholesteryl ester transfer protein (CETP) inhibition: beyond raising high-density lipoprotein cholesterol levels: pathways by which modulation of CETP may alter atherogenesis. Arterioscler. Thromb. Vasc. Biol. 26, 706–715 (2006).
Mahley, R.W., Huang, Y. & Weisgraber, K.H. Putting cholesterol in its place: apoE and reverse cholesterol transport. J. Clin. Invest. 116, 1226–1229 (2006).
Bruce, C., Beamer, L.J. & Tall, A.R. The implications of the structure of the bactericidal/permeability-increasing protein on the lipid-transfer function of the cholesteryl ester transfer protein. Curr. Opin. Struct. Biol. 8, 426–434 (1998).
Beamer, L.J. Structure of human BPI (bactericidal/permeability-increasing protein) and implications for related proteins. Biochem. Soc. Trans. 31, 791–794 (2003).
Beamer, L.J., Carroll, S.F. & Eisenberg, D. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science 276, 1861–1864 (1997).
Hamilton, J.A. Fatty acid interactions with proteins: what X-ray crystal and NMR solution structures tell us. Prog. Lipid Res. 43, 177–199 (2004).
Alpy, F. & Tomasetto, C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J. Cell Sci. 118, 2791–2801 (2005).
Malinina, L., Malakhova, M.L., Teplov, A., Brown, R.E. & Patel, D.J. Structural basis for glycosphingolipid transfer specificity. Nature 430, 1048–1053 (2004).
Im, Y.J., Raychaudhuri, S., Prinz, W.A. & Hurley, J.H. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature 437, 154–158 (2005).
Sha, B., Phillips, S.E., Bankaitis, V.A. & Luo, M. Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature 391, 506–510 (1998).
Qiu, X. & Janson, C.A. Structure of apo acyl carrier protein and a proposal to engineer protein crystallization through metal ions. Acta Crystallogr. D Biol. Crystallogr. 60, 1545–1554 (2004).
Connolly, D.T. et al. Physical and kinetic characterization of recombinant human cholesteryl ester transfer protein. Biochem. J. 320, 39–47 (1996).
Clark, R.W., Ruggeri, R.B., Cunningham, D. & Bamberger, M.J. Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J. Lipid Res. 47, 537–552 (2006).
Wright, C.S., Li, S.C. & Rastinejad, F. Crystal structure of human GM2-activator protein with a novel β-cup topology. J. Mol. Biol. 304, 411–422 (2000).
Wang, S., Kussie, P., Deng, L. & Tall, A. Defective binding of neutral lipids by a carboxyl-terminal deletion mutant of cholesteryl ester transfer protein. Evidence for a carboxyl-terminal cholesteryl ester binding site essential for neutral lipid transfer activity. J. Biol. Chem. 270, 612–618 (1995).
Rajaram, O.V. & Sawyer, W.H. Penetration of an emulsion surface by cholesteryl transfer protein. Eur. Biophys. J. 25, 31–36 (1996).
Zheng, K.Q. et al. A novel missense mutation (L296Q) in cholesteryl ester transfer protein gene related to coronary heart disease. Acta Biochim. Biophys. Sin. (Shanghai) 36, 33–36 (2004).
Bruce, C. et al. Molecular determinants of plasma cholesteryl ester transfer protein binding to high density lipoproteins. J. Biol. Chem. 270, 11532–11542 (1995).
Jiang, X.C. et al. Point mutagenesis of positively charged amino acids of cholesteryl ester transfer protein: conserved residues within the lipid transfer/lipopolysaccharide binding protein gene family essential for function. Biochemistry 34, 7258–7263 (1995).
Ohnishi, T., Oikawa, K., Kay, C.M. & Yokoyama, S. Modulation of substrate selectivity in plasma lipid transfer protein reaction over structural variation of lipid particle. Biochim. Biophys. Acta 1254, 117–126 (1995).
Serdyuk, A.P. & Morton, R.E. Lipid transfer inhibitor protein defines the participation of lipoproteins in lipid transfer reactions: CETP has no preference for cholesteryl esters in HDL versus LDL. Arterioscler. Thromb. Vasc. Biol. 19, 718–726 (1999).
Peter, B.J. et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499 (2004).
Miller, K.W. & Small, D.M. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J. Biol. Chem. 258, 13772–13784 (1983).
Hamilton, J.A. & Small, D.M. Solubilization and localization of cholesteryl oleate in egg phosphatidylcholine vesicles. A carbon 13 NMR study. J. Biol. Chem. 257, 7318–7321 (1982).
Hamilton, J.A., Miller, K.W. & Small, D.M. Solubilization of triolein and cholesteryl oleate in egg phosphatidylcholine vesicles. J. Biol. Chem. 258, 12821–12826 (1983).
Morton, R.E. & Steinbrunner, J.V. Concentration of neutral lipids in the phospholipid surface of substrate particles determines lipid transfer protein activity. J. Lipid Res. 31, 1559–1567 (1990).
Morton, R.E. & Zilversmit, D.B. Inter-relationship of lipids transferred by the lipid-transfer protein isolated from human lipoprotein-deficient plasma. J. Biol. Chem. 258, 11751–11757 (1983).
Ko, K.W., Ohnishi, T. & Yokoyama, S. Triglyceride transfer is required for net cholesteryl ester transfer between lipoproteins in plasma by lipid transfer protein. Evidence for a hetero-exchange transfer mechanism demonstrated by using novel monoclonal antibodies. J. Biol. Chem. 269, 28206–28213 (1994).
Urlaub, G., Kas, E., Carothers, A.M. & Chasin, L.A. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell 33, 405–412 (1983).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Lloyd, D.B. et al. Cholesteryl ester transfer protein variants have differential stability but uniform inhibition by torcetrapib. J. Biol. Chem. 280, 14918–14922 (2005).
Acknowledgements
We thank the staffs in the X-ray group and synchrotrons for assistance in data collection, G. Andrews, M. Bamberger, L. Morehouse, D. Perry, R. Ruggeri, K. Ranney, I. Reininger, M. Tu and P. Zagouras for insightful discussions and S. Liu, J. Boyd, D. Cunningham, T. Dickinson, J. Duerr, B. King, T. Lanzetti, W. Lin, P. Loulakis, M. Mansour, A. McColl, T. McLellan, F. Rajamohan, M. Rosner, M. Tardie and Z. Xie for supporting work.
Author information
Authors and Affiliations
Contributions
M.C.G., S.J.H., T.A.S., I.-K.W., H.Z., K.M.M., K.J.S.-E., T.B.F., L.R.H., K.F.G., Y.C., G.A.K., B.A.C., J.S.C. and A.K.S. provided key reagents. A.M., M.J.A. and D.E.D. crystallized the proteins. X.Q. solved the structure. M.E.L., D.B.L., J.F.T. and R.W.C. contributed to mutagenesis and assays. X.Q., P.H., C.M.H. and A.P.S coordinated the research and data review. X.Q., J.F.T., K.F.G. and A.P.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors are employees of Pfizer Inc., which develops and markets medicines to treat cardiovascular disease.
Supplementary information
Supplementary Fig. 1
Structure-based alignment of human CETP species and BPI. (PDF 436 kb)
Supplementary Data
Additional methods and data. (PDF 165 kb)
Rights and permissions
About this article
Cite this article
Qiu, X., Mistry, A., Ammirati, M. et al. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol 14, 106–113 (2007). https://doi.org/10.1038/nsmb1197
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1197
This article is cited by
-
Discovery of novel cholesteryl ester transfer protein (CETP) inhibitors by a multi-stage virtual screening
BMC Chemistry (2024)
-
Structure-based mechanism and inhibition of cholesteryl ester transfer protein
Current Atherosclerosis Reports (2023)
-
Risk prediction of nephropathy by integrating clinical and genetic information among adult patients with type 2 diabetes
Acta Diabetologica (2022)
-
Peptide VSAK maintains tissue glucose uptake and attenuates pro-inflammatory responses caused by LPS in an experimental model of the systemic inflammatory response syndrome: a PET study
Scientific Reports (2021)
-
The Role of Phospholipid Transfer Protein in the Development of Atherosclerosis
Current Atherosclerosis Reports (2021)