Abstract
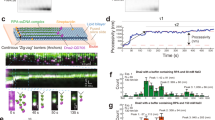
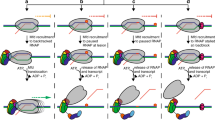
Helicases unwind RNA or DNA duplexes and displace proteins from nucleic acids in an ATP-dependent fashion. To unwind duplexes, helicases typically load onto one of the two nucleic acid strands, usually at a single-stranded region, and then translocate on this strand in a unidirectional fashion, thereby displacing the complementary DNA or RNA. Here we show that the DEAD-box RNA helicase Ded1 unwinds duplexes in a different manner. Ded1 uses the single-stranded region to gain access to the duplex. Strand separation is directly initiated from the duplex region and no covalent connection between the single strand and the duplex region is required. This new type of helicase activity explains observations with other DEAD-box proteins and may be the prototype for duplex-unwinding reactions in RNA metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Cordin, O., Banroques, J., Tanner, N.K. & Linder, P. The DEAD-box protein family of RNA helicases. Gene 367, 17–37 (2006).
Eoff, R.L. & Raney, K.D. Helicase-catalysed translocation and strand separation. Biochem. Soc. Trans. 33, 1474–1478 (2005).
Caruthers, J.M. & McKay, D.B. Helicase structure and mechanism. Curr. Opin. Struct. Biol. 12, 123–133 (2002).
Tanner, N.K. & Linder, P. DExD/H box RNA helicases. From generic motors to specific dissociation functions. Mol. Cell 8, 251–261 (2001).
Bowers, H.A. et al. Discriminatory RNP remodeling by the DEAD-box protein DED1. RNA 12, 903–912 (2006).
Fairman, M.E. et al. Protein displacement by DExH/D RNA helicases without duplex unwinding. Science 304, 730–734 (2004).
Linder, P. The life of RNA with proteins. Science 304, 694–695 (2004).
Will, C.L. & Luehrmann, R. RNP remodeling with DExH/D boxes. Science 291, 1916–1917 (2001).
Byrd, A.K. & Raney, K.D. Protein displacement by an assembly of helicase molecules aligned along single-stranded DNA. Nat. Struct. Mol. Biol. 11, 531–538 (2004).
Dumont, S. et al. RNA translocation and unwinding mechanism of HCV NS3 helicase and its coordination by ATP. Nature 439, 105–108 (2006).
Jankowsky, E., Gross, C.H., Shuman, S. & Pyle, A.M. The DExH protein NPH-II is a processive and directional motor for unwinding RNA. Nature 403, 447–451 (2000).
Myong, S., Rasnik, I., Joo, C., Lohman, T.M. & Ha, T. Repetitive shuttling of a motor protein on DNA. Nature 437, 1321–1325 (2005).
Kawaoka, J., Jankowsky, E. & Pyle, A.M. Backbone tracking by the SF2 helicase NPH-II. Nat. Struct. Mol. Biol. 11, 526–530 (2004).
von Hippel, P.H. Helicases become mechanistically simpler and functionally more complex. Nat. Struct. Mol. Biol. 11, 494–496 (2004).
Lohman, T.M. & Bjornson, K.P. Mechanisms of helicase-catalyzed DNA unwinding. Annu. Rev. Biochem. 65, 169–214 (1996).
Jankowsky, E., Fairman, M.E. & Yang, Q. RNA helicases: versatile ATP-driven nanomotors. J. Nanosci. Nanotechnol. 5, 1983–1989 (2005).
Rogers, G.W.J., Lima, W.F. & Merrick, W.C. Further characterization of the helicase activity of eIF4A. Substrate specificity. J. Biol. Chem. 276, 12598–12608 (2001).
Huang, Y. & Liu, Z.R. The ATPase, RNA unwinding, and RNA binding activities of recombinant p68 RNA helicase. J. Biol. Chem. 277, 12810–12815 (2002).
Bizebard, T., Ferlenghi, I., Iost, I. & Dreyfus, M. Studies on three E. coli DEAD-box helicases point to an unwinding mechanism different from that of model DNA helicases. Biochemistry 43, 7857–7866 (2004).
Linder, P. Yeast RNA helicases of the DEAD-box family involved in translation initiation. Biol. Cell 95, 157–167 (2003).
Chuang, R.Y., Weaver, P.L., Liu, Z. & Chang, T.H. Requirement of the DEAD-Box protein ded1p for messenger RNA translation. Science 275, 1468–1471 (1997).
Iost, I., Dreyfus, M. & Linder, P. Ded1p, a DEAD-box protein required for translation initiation in Saccharomyces cerevisiae, is an RNA helicase. J. Biol. Chem. 274, 17677–17683 (1999).
Yang, Q. & Jankowsky, E. ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry 44, 13591–13601 (2005).
Singleton, M.R., Dillingham, M.S., Gaudier, M., Kowalczykowski, S.C. & Wigley, D.B. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature 432, 187–193 (2004).
Dillingham, M.S., Spies, M. & Kowalczykowski, S.C. RecBCD enzyme is a bipolar DNA helicase. Nature 423, 893–897 (2003).
Cordin, O., Tanner, N.K., Doere, M., Linder, P. & Banroques, J. The newly discovered Q motif of DEAD-box RNA helicases regulates RNA-binding and helicase activity. EMBO J. 23, 2478–2487 (2004).
Tanaka, N. & Schwer, B. Characterization of the NTPase, RNA-binding, and RNA helicase activities of the DEAH-box splicing factor Prp22. Biochemistry 44, 9795–9803 (2005).
Tanaka, N. & Schwer, B. Mutations in PRP43 that uncouple RNA-dependent NTPase activity and pre-mRNA splicing function. Biochemistry 45, 6510–6521 (2006).
Kawaoka, J. & Pyle, A.M. Choosing between DNA and RNA: the polymer specificity of RNA helicase NPH-II. Nucleic Acids Res. 33, 644–649 (2005).
Shuman, S. Vaccinia virus RNA helicase: an essential enzyme related to the DE-H family of RNA-dependent NTPases. Proc. Natl. Acad. Sci. USA 89, 10935–10939 (1992).
Shuman, S. Vaccinia virus RNA helicase. Directionality and substrate specificity. J. Biol. Chem. 268, 11798–11802 (1993).
Beran, R.K., Bruno, M.M., Bowers, H.A., Jankowsky, E. & Pyle, A.M. Robust translocation along a molecular monorail: the NS3 helicase from hepatitis C virus traverses unusually large disruptions in its track. J. Mol. Biol. 358, 974–982 (2006).
Bianco, P.R. & Kowalczykowski, S.C. Translocation step size and mechanism of the RecBC DNA helicase. Nature 405, 368–372 (2000).
Rocak, S. & Linder, P. DEAD-box proteins: the driving forces behind RNA metabolism. Nat. Rev. Mol. Cell Biol. 5, 232–241 (2004).
Sengoku, T., Nureki, O., Nakamura, A., Kobayashi, S. & Yokoyama, S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 125, 287–300 (2006).
Bono, F., Ebert, J., Lorentzen, E. & Conti, E. The crystal structure of the exon junction complex reveals how it maintains a stable grip on mRNA. Cell 126, 713–725 (2006).
Serebrov, V. & Pyle, A.M. Periodic cycles of RNA unwinding and pausing by hepatitis C virus NS3 helicase. Nature 430, 476–480 (2004).
Acknowledgements
We thank M. Fairman for purification of NPH-II and A. Pyle, T. Nilsen, W. Merrick, J. Coller, M. Caprara and N. Kaye for comments on the manuscript. This work was supported by a grant from the US National Institutes of Health to E.J. E.J. is a Scholar of the Damon Runyon Cancer Research Foundation.
Author information
Authors and Affiliations
Contributions
Q.Y. conducted experiments. Q.Y. and E.J. conceived and planned experiments, analyzed and interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Unwinding of MPS III components without streptavidin. (PDF 46 kb)
Supplementary Fig. 2
Unwinding reactions under single-turnover conditions. (PDF 169 kb)
Supplementary Fig. 3
Representative time courses for duplex unwinding. (PDF 43 kb)
Supplementary Fig. 4
Sequences of substrates used in Figures 1, 2, 3. (PDF 13 kb)
Supplementary Fig. 5
Unwinding of the 16-bp blunt-end RNA duplex requires ATP hydrolysis. (PDF 51 kb)
Supplementary Fig. 6
Sequences and functional characterization of substrates used in Figure 4. (PDF 67 kb)
Supplementary Fig. 7
Sequences of multipiece substrates used in Figures 5, 6, 7. (PDF 16 kb)
Rights and permissions
About this article
Cite this article
Yang, Q., Jankowsky, E. The DEAD-box protein Ded1 unwinds RNA duplexes by a mode distinct from translocating helicases. Nat Struct Mol Biol 13, 981–986 (2006). https://doi.org/10.1038/nsmb1165
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1165
This article is cited by
-
Cellular functions of eukaryotic RNA helicases and their links to human diseases
Nature Reviews Molecular Cell Biology (2023)
-
NF90 interacts with components of RISC and modulates association of Ago2 with mRNA
BMC Biology (2022)
-
The RNA helicase Mtr4p is a duplex-sensing translocase
Nature Chemical Biology (2017)
-
Plasmodium falciparum specific helicase 3 is nucleocytoplasmic protein and unwinds DNA duplex in 3′ to 5′ direction
Scientific Reports (2017)
-
DEAD-box protein DDX3 associates with eIF4F to promote translation of selected mRNAs
The EMBO Journal (2012)