Abstract
Apolipoprotein (apo)A-I is an organizing scaffold protein that is critical to high-density lipoprotein (HDL) structure and metabolism, probably mediating many of its cardioprotective properties. However, HDL biogenesis is poorly understood, as lipid-free apoA-I has been notoriously resistant to high-resolution structural study. Published models from low-resolution techniques share certain features but vary considerably in shape and secondary structure. To tackle this central issue in lipoprotein biology, we assembled a team of structural biologists specializing in apolipoproteins and set out to build a consensus model of monomeric lipid-free human apoA-I. Combining novel and published cross-link constraints, small-angle X-ray scattering (SAXS), hydrogen–deuterium exchange (HDX) and crystallography data, we propose a time-averaged model consistent with much of the experimental data published over the last 40 years. The model provides a long-sought platform for understanding and testing details of HDL biogenesis, structure and function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Soutar, A.K. et al. Effect of the human plasma apolipoproteins and phosphatidylcholine acyl donor on the activity of lecithin: cholesterol acyltransferase. Biochemistry 14, 3057–3064 (1975).
Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 289, 24020–24029 (2014).
Borhani, D.W., Engler, J.A. & Brouillette, C.G. Crystallization of truncated human apolipoprotein A-I in a novel conformation. Acta Crystallogr. D Biol. Crystallogr. 55, 1578–1583 (1999).
Mei, X. & Atkinson, D. Crystal structure of C-terminal truncated apolipoprotein A-I reveals the assembly of high density lipoprotein (HDL) by dimerization. J. Biol. Chem. 286, 38570–38582 (2011).
Melchior, J.T. et al. An evaluation of the crystal structure of C-terminal truncated apolipoprotein A-I in solution reveals structural dynamics related to lipid binding. J. Biol. Chem. 291, 5439–5451 (2016).
Nolte, R.T. & Atkinson, D. Conformational analysis of apolipoprotein A-I and E-3 based on primary sequence and circular dichroism. Biophys. J. 63, 1221–1239 (1992).
Barbeau, D.L., Jonas, A., Teng, T. & Scanu, A.M. Asymmetry of apolipoprotein A-I in solution as assessed from ultracentrifugal, viscometric, and fluorescence polarization studies. Biochemistry 18, 362–369 (1979).
Okon, M., Frank, P.G., Marcel, Y.L. & Cushley, R.J. Heteronuclear NMR studies of human serum apolipoprotein A-I. Part I. Secondary structure in lipid-mimetic solution. FEBS Lett. 517, 139–143 (2002).
Davidson, W.S. et al. Structural organization of the N-terminal domain of apolipoprotein A-I: studies of tryptophan mutants. Biochemistry 38, 14387–14395 (1999).
Segrest, J.P., Jones, M.K., Shao, B. & Heinecke, J.W. An experimentally robust model of monomeric apolipoprotein A-I created from a chimera of two X-ray structures and molecular dynamics simulations. Biochemistry 53, 7625–7640 (2014).
Silva, R.A., Hilliard, G.M., Fang, J., Macha, S. & Davidson, W.S. A three-dimensional molecular model of lipid-free apolipoprotein A-I determined by cross-linking/mass spectrometry and sequence threading. Biochemistry 44, 2759–2769 (2005).
Chetty, P.S. et al. Helical structure and stability in human apolipoprotein A-I by hydrogen exchange and mass spectrometry. Proc. Natl. Acad. Sci. USA 106, 19005–19010 (2009).
Lagerstedt, J.O. et al. The “beta-clasp” model of apolipoprotein A-I--a lipid-free solution structure determined by electron paramagnetic resonance spectroscopy. Biochim. Biophys. Acta 1821, 448–455 (2012).
Oda, M.N., Forte, T.M., Ryan, R.O. & Voss, J.C. The C-terminal domain of apolipoprotein A-I contains a lipid-sensitive conformational trigger. Nat. Struct. Biol. 10, 455–460 (2003).
Phillips, M.C. New insights into the determination of HDL structure by apolipoproteins: Thematic review series: high density lipoprotein structure, function, and metabolism. J. Lipid Res. 54, 2034–2048 (2013).
Pollard, R.D., Fulp, B., Samuel, M.P., Sorci-Thomas, M.G. & Thomas, M.J. The conformation of lipid-free human apolipoprotein A-I in solution. Biochemistry 52, 9470–9481 (2013).
Zhang, X., Lei, D., Zhang, L., Rames, M. & Zhang, S. A model of lipid-free apolipoprotein A-I revealed by iterative molecular dynamics simulation. PLoS One 10, e0120233 (2015).
Swaim, C.L., Smith, J.B. & Smith, D.L. Unexpected products from the reaction of the synthetic cross-linker 3,3′-dithiobis(sulfosuccinimidyl propionate), DTSSP with peptides. J. Am. Soc. Mass Spectrom. 15, 736–749 (2004).
Leavell, M.D., Novak, P., Behrens, C.R., Schoeniger, J.S. & Kruppa, G.H. Strategy for selective chemical cross-linking of tyrosine and lysine residues. J. Am. Soc. Mass Spectrom. 15, 1604–1611 (2004).
Walker, R.G. et al. The structure of human apolipoprotein A-IV as revealed by stable isotope-assisted cross-linking, molecular dynamics, and small angle x-ray scattering. J. Biol. Chem. 289, 5596–5608 (2014).
Franke, D. & Svergun, D.I. DAMMIF, a program for rapid ab-initio shape determination in small-angle scattering. J. Appl. Crystallogr. 42, 342–346 (2009).
Krieger, E. et al. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 77 (Suppl. 9), 114–122 (2009).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Willard, L. et al. VADAR: a web server for quantitative evaluation of protein structure quality. Nucleic Acids Res. 31, 3316–3319 (2003).
de la Llera-Moya, M. et al. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler. Thromb. Vasc. Biol. 30, 796–801 (2010).
Vedhachalam, C. et al. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler. Thromb. Vasc. Biol. 27, 1603–1609 (2007).
Hassan, H.H. et al. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J. Lipid Res. 48, 2428–2442 (2007).
Rye, K.A. & Barter, P.J. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24, 421–428 (2004).
Vedhachalam, C. et al. Influence of ApoA-I structure on the ABCA1-mediated efflux of cellular lipids. J. Biol. Chem. 279, 49931–49939 (2004).
Saito, H. et al. Domain structure and lipid interaction in human apolipoproteins A-I and E, a general model. J. Biol. Chem. 278, 23227–23232 (2003).
Mei, X., Liu, M., Herscovitz, H. & Atkinson, D. Probing the C-terminal domain of lipid-free apoA-I demonstrates the vital role of the H10B sequence repeat in HDL formation. J. Lipid Res. 57, 1507–1517 (2016).
Koyama, M. et al. Interaction between the N- and C-terminal domains modulates the stability and lipid binding of apolipoprotein A-I. Biochemistry 48, 2529–2537 (2009).
Gorshkova, I.N., Liadaki, K., Gursky, O., Atkinson, D. & Zannis, V.I. Probing the lipid-free structure and stability of apolipoprotein A-I by mutation. Biochemistry 39, 15910–15919 (2000).
Gross, E., Peng, D.Q., Hazen, S.L. & Smith, J.D. A novel folding intermediate state for apolipoprotein A-I: role of the amino and carboxy termini. Biophys. J. 90, 1362–1370 (2006).
Pollard, R.D., Fulp, B., Sorci-Thomas, M.G. & Thomas, M.J. High-density lipoprotein biogenesis: defining the domains involved in human apolipoprotein A-I lipidation. Biochemistry 55, 4971–4981 (2016).
Panagotopulos, S.E. et al. The role of apolipoprotein A-I helix 10 in apolipoprotein-mediated cholesterol efflux via the ATP-binding cassette transporter ABCA1. J. Biol. Chem. 277, 39477–39484 (2002).
Brouillette, C.G. et al. Förster resonance energy transfer measurements are consistent with a helical bundle model for lipid-free apolipoprotein A-I. Biochemistry 44, 16413–16425 (2005).
Puchkaev, A.V., Koo, L.S. & Ortiz de Montellano, P.R. Aromatic stacking as a determinant of the thermal stability of CYP119 from Sulfolobus solfataricus. Arch. Biochem. Biophys. 409, 52–58 (2003).
Nichols, W.C., Dwulet, F.E., Liepnieks, J. & Benson, M.D. Variant apolipoprotein AI as a major constituent of a human hereditary amyloid. Biochem. Biophys. Res. Commun. 156, 762–768 (1988).
Franceschini, G., Sirtori, C.R., Capurso, A. II, Weisgraber, K.H. & Mahley, R.W. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J. Clin. Invest. 66, 892–900 (1980).
Chetty, P.S. et al. Effects of the Iowa and Milano mutations on apolipoprotein A-I structure and dynamics determined by hydrogen exchange and mass spectrometry. Biochemistry 51, 8993–9001 (2012).
Gorshkova, I.N. et al. Structure and stability of apolipoprotein a-I in solution and in discoidal high-density lipoprotein probed by double charge ablation and deletion mutation. Biochemistry 45, 1242–1254 (2006).
Davidson, W.S., Hazlett, T., Mantulin, W.W. & Jonas, A. The role of apolipoprotein AI domains in lipid binding. Proc. Natl. Acad. Sci. USA 93, 13605–13610 (1996).
Saito, H. et al. Alpha-helix formation is required for high affinity binding of human apolipoprotein A-I to lipids. J. Biol. Chem. 279, 20974–20981 (2004).
Tanaka, M. et al. Influence of N-terminal helix bundle stability on the lipid-binding properties of human apolipoprotein A-I. Biochim. Biophys. Acta 1811, 25–30 (2011).
Pownall, H.J., Massey, J.B., Kusserow, S.K. & Gotto, A.M. Jr. Kinetics of lipid--protein interactions: interaction of apolipoprotein A-I from human plasma high density lipoproteins with phosphatidylcholines. Biochemistry 17, 1183–1188 (1978).
Palgunachari, M.N. et al. Only the two end helixes of eight tandem amphipathic helical domains of human apo A-I have significant lipid affinity. Implications for HDL assembly. Arterioscler. Thromb. Vasc. Biol. 16, 328–338 (1996).
Qian, H. et al. Structure of the human lipid exporter ABCA1. Cell 169, 1228–1239.e10 (2017).
Segrest, J.P. et al. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J. Biol. Chem. 274, 31755–31758 (1999).
Huang, B.X., Kim, H.Y. & Dass, C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass Spectrom. 15, 1237–1247 (2004).
Jacobsen, R.B. et al. Structure and dynamics of dark-state bovine rhodopsin revealed by chemical cross-linking and high-resolution mass spectrometry. Protein Sci. 15, 1303–1317 (2006).
Young, M.M. et al. High throughput protein fold identification by using experimental constraints derived from intramolecular cross-links and mass spectrometry. Proc. Natl. Acad. Sci. USA 97, 5802–5806 (2000).
Peng, L., Rasmussen, M.I., Chailyan, A., Houen, G. & Højrup, P. Probing the structure of human protein disulfide isomerase by chemical cross-linking combined with mass spectrometry. J. Proteomics 108, 1–16 (2014).
Tubb, M.R., Smith, L.E. & Davidson, W.S. Purification of recombinant apolipoproteins A-I and A-IV and efficient affinity tag cleavage by tobacco etch virus protease. J. Lipid Res. 50, 1497–1504 (2009).
Markwell, M.A., Haas, S.M., Bieber, L.L. & Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87, 206–210 (1978).
Petrotchenko, E.V., Serpa, J.J. & Borchers, C.H. An isotopically coded CID-cleavable biotinylated cross-linker for structural proteomics. Mol. Cell Proteomics 10, M110.001420 (2011).
Lima, D.B. et al. SIM-XL: A powerful and user-friendly tool for peptide cross-linking analysis. J. Proteomics 129, 51–55 (2015).
Dyer, K.N. et al. High-throughput SAXS for the characterization of biomolecules in solution: a practical approach. Methods Mol. Biol. 1091, 245–258 (2014).
Borhani, D.W., Rogers, D.P., Engler, J.A. & Brouillette, C.G. Crystal structure of truncated human apolipoprotein A-I suggests a lipid-bound conformation. Proc. Natl. Acad. Sci. USA 94, 12291–12296 (1997).
Sali, A. & Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Schneidman-Duhovny, D., Hammel, M. & Sali, A. FoXS: a web server for rapid computation and fitting of SAXS profiles. Nucleic Acids Res. 38, W540–W544 (2010).
Schneidman-Duhovny, D., Hammel, M., Tainer, J.A. & Sali, A. Accurate SAXS profile computation and its assessment by contrast variation experiments. Biophys. J. 105, 962–974 (2013).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
This work was supported by an American Heart Association postdoctoral fellowship grant (16POST27710016 to J.T.M.), an National Institutes of Health Heart Lung and Blood Institute funded predoctoral fellowship to M.C. (HL125204-03), R01 GM098458 to W.S.D. and T.B.T., R01 HL112276 and HL127649 to M.G.S.-T., P01 HL026335 and R01 HL116518 to D.A., P01 HL12803 to W.S.D., J.P.S. and J.W.H. The MS data was acquired in the University of Cincinnati Proteomics Laboratory under the direction of K. Greis on a mass spectrometer funded in part through an NIH S10 shared instrumentation grant (RR027015 Gries-PI).
Author information
Authors and Affiliations
Contributions
J.T.M. and W.S.D. conceived and designed new experiments reported in this paper. J.T.M., R.G.W., A.L.C., J.M., M.C. and H.D.S. performed experiments. J.T.M., R.G.W., M.C., T.B.T., M.K.J., H.D.S., J.P.S., M.C.P. and W.S.D. analyzed data. J.T.M., M.C., T.B.T., M.K.J., H.D.S., K.-A.R., M.N.O., M.G.S.-T., M.J.T., J.W.H., X.M., D.A., J.P.S., S.L.-K., M.C.P. and W.S.D. derived the model. J.T.M., T.B.T., K.-A.R., M.N.O., M.G.S.-T., M.J.T., J.W.H., D.A., J.P.S., S.L.-K., M.C.P. and W.S.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
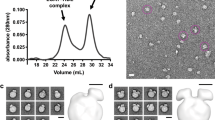
Supplementary Figure 1 Separation and purification of lipid-free apoA-I monomer by gel filtration chromatography
ApoA-I was cross-linked and subjected to gel-filtration chromatography and fractions corresponding to the stable monomeric species were pooled. Chromatograms of apoA-I cross-linked with CBDPS and BS3 are shown in panels (a) and (c), respectively. The shaded area represents the fractions corresponding to monomeric apoA-I that were pooled for cross-linking and SAXS analysis. Corresponding SDS-PAGE analysis of lipid-free apoA-I cross-linked with CBPDS and BS3 are shown in panels (b) and (d), respectively. Molecular weight markers are shown in lane 1, cross-linked apoA-I prior to separation is shown in lane 2, and cross-linked monomeric apoA-I after separation is shown in lane 3. Gels were stained with coomassie blue.
Supplementary Figure 2 Derivation of an all atom model of full-length, lipid-free monomeric apoA-I
Panel (a) shows a single molecule from the reported crystal structure of the apoA-I1–184 dimer. Panel (b) shows the folding of helix 6 previously proposed by Mei et. al.34, and the fold of helix 6 used for the time-averaged structure (right). Panel (c) shows the final time-averaged model. Molecules are colored as previously defined by Mei et. al.34. Purple and cyan represent consensus sequence peptide A and B homology sequences, green represents exon-3-encoded region (residues 1–43) and yellow are prolines.
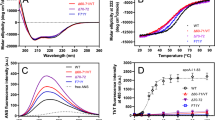
Supplementary Figure 3 Comparison of the newest model to previous models with respect to various pieces of experimental data
The line diagrams show the fit of the models relative to the target value (black circle) derived from current and previous data on lipid-free monomeric apoA-I. Panel (a) shows the model fits to experimental cross-links from the universal cross-linking list (Supplemental Table 4) with the target being zero violations. Panel (b) shows the model fits to experimental H-DX data with the target being zero violations. Panel (c) shows the averaged χ2 values for all models fit to the scattering profiles derived from apoA-I cross-linked with BS3 and CBDPS. The target for SAXS is the lowest χ2 value possible with lower values indicating better fits to the experimental scattering curve. Panel (d) shows the fits to overall α-helical data derived values reported across 27 studies as shown in Supplementary Table 5. Panel (e) shows the rank of the MolProbity score of all reported models of apoA-I relative to 27,675 crystal structures reported in the protein database.
Supplementary Figure 4 Effect of temperature on H-DX in lipid-free apoA-I.
The plots compare the measured H-DX kinetics of the apoA-I peptide 159-169 from a helical region at pD 7 and (A) 5°C, (B) 25°C and (C) 37°C to the rate for the peptide in a dynamically disordered state (dashed line). Comparison of the rate constants derived by fitting the dashed and solid time-courses to mono-exponential rate equations yields the protection factor (Pf) and hence the free energy (ΔG) of helix stabilization. After correcting for the effect of temperature on the intrinsic chemical HX rate, the apparent ΔG of helix stability at 5°C and 25°C is 5.3 and 3.8 kcal/mol, respectively. The helix stability is less at 37°C and H-D exchange is complete in ~3 min. [From26]
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1–7 and Supplementary Note (PDF 1583 kb)
Rights and permissions
About this article
Cite this article
Melchior, J., Walker, R., Cooke, A. et al. A consensus model of human apolipoprotein A-I in its monomeric and lipid-free state. Nat Struct Mol Biol 24, 1093–1099 (2017). https://doi.org/10.1038/nsmb.3501
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3501
This article is cited by
-
Opportunities, challenges, and benefits of AI innovation in government services: a review
Discover Artificial Intelligence (2024)
-
Analyses of familial chylomicronemia syndrome in Pereira, Colombia 2010–2020: a cross-sectional study
Lipids in Health and Disease (2023)
-
Amyloidogenic 60–71 deletion/ValThr insertion mutation of apolipoprotein A-I generates a new aggregation-prone segment that promotes nucleation through entropic effects
Scientific Reports (2023)
-
Serum apolipoprotein A-I potentiates the therapeutic efficacy of lysocin E against Staphylococcus aureus
Nature Communications (2021)
-
Structure–function analysis of naturally occurring apolipoprotein A-I L144R, A164S and L178P mutants provides insight on their role on HDL levels and cardiovascular risk
Cellular and Molecular Life Sciences (2021)