Abstract
Directional translocation of the ribosome through the mRNA open reading frame is a critical determinant of translational fidelity. This process entails a complex interplay of large-scale conformational changes within the actively translating particle, which together coordinate the movement of tRNA and mRNA substrates with respect to the large and small ribosomal subunits. Using pre–steady state, single-molecule fluorescence resonance energy transfer imaging, we tracked the nature and timing of these conformational events within the Escherichia coli ribosome from five structural perspectives. Our investigations revealed direct evidence of structurally and kinetically distinct late intermediates during substrate movement, whose resolution determines the rate of translocation. These steps involve intramolecular events within the EF-G–GDP–bound ribosome, including exaggerated, reversible fluctuations of the small-subunit head domain, which ultimately facilitate peptidyl-tRNA's movement into its final post-translocation position.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Agirrezabala, X. & Frank, J. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Q. Rev. Biophys. 42, 159–200 (2009).
Holtkamp, W., Wintermeyer, W. & Rodnina, M.V. Synchronous tRNA movements during translocation on the ribosome are orchestrated by elongation factor G and GTP hydrolysis. BioEssays 36, 908–918 (2014).
Moore, P.B. How should we think about the ribosome? Annu. Rev. Biophys. 41, 1–19 (2012).
Voorhees, R.M. & Ramakrishnan, V. Structural basis of the translational elongation cycle. Annu. Rev. Biochem. 82, 203–236 (2013).
Dinman, J.D. Mechanisms and implications of programmed translational frameshifting. Wiley Interdiscip. Rev. RNA 3, 661–673 (2012).
Munro, J.B., Sanbonmatsu, K.Y., Spahn, C.M. & Blanchard, S.C. Navigating the ribosome's metastable energy landscape. Trends Biochem. Sci. 34, 390–400 (2009).
Shoji, S., Walker, S.E. & Fredrick, K. Ribosomal translocation: one step closer to the molecular mechanism. ACS Chem. Biol. 4, 93–107 (2009).
Chen, J., Tsai, A., O'Leary, S.E., Petrov, A. & Puglisi, J.D. Unraveling the dynamics of ribosome translocation. Curr. Opin. Struct. Biol. 22, 804–814 (2012).
Spirin, A.S. On the mechanism of ribosome function: the hypothesis of locking-unlocking of subparticles. Dokl. Akad. Nauk SSSR 179, 1467–1470 (1968).
Peske, F., Matassova, N.B., Savelsbergh, A., Rodnina, M.V. & Wintermeyer, W. Conformationally restricted elongation factor G retains GTPase activity but is inactive in translocation on the ribosome. Mol. Cell 6, 501–505 (2000).
Horan, L.H. & Noller, H.F. Intersubunit movement is required for ribosomal translocation. Proc. Natl. Acad. Sci. USA 104, 4881–4885 (2007).
Munro, J.B., Wasserman, M.R., Altman, R.B., Wang, L. & Blanchard, S.C. Correlated conformational events in EF-G and the ribosome regulate translocation. Nat. Struct. Mol. Biol. 17, 1470–1477 (2010).
Guo, Z. & Noller, H.F. Rotation of the head of the 30S ribosomal subunit during mRNA translocation. Proc. Natl. Acad. Sci. USA 109, 20391–20394 (2012).
Cornish, P.V., Ermolenko, D.N., Noller, H.F. & Ha, T. Spontaneous intersubunit rotation in single ribosomes. Mol. Cell 30, 578–588 (2008).
Ermolenko, D.N. & Noller, H.F. mRNA translocation occurs during the second step of ribosomal intersubunit rotation. Nat. Struct. Mol. Biol. 18, 457–462 (2011).
Ermolenko, D.N. et al. Observation of intersubunit movement of the ribosome in solution using FRET. J. Mol. Biol. 370, 530–540 (2007).
Munro, J.B., Altman, R.B., O'Connor, N. & Blanchard, S.C. Identification of two distinct hybrid state intermediates on the ribosome. Mol. Cell 25, 505–517 (2007).
Munro, J.B. et al. Spontaneous formation of the unlocked state of the ribosome is a multistep process. Proc. Natl. Acad. Sci. USA 107, 709–714 (2010).
Fei, J., Kosuri, P., MacDougall, D.D. & Gonzalez, R.L. Jr. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol. Cell 30, 348–359 (2008).
Ratje, A.H. et al. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature 468, 713–716 (2010).
Ramrath, D.J. et al. Visualization of two transfer RNAs trapped in transit during elongation factor G-mediated translocation. Proc. Natl. Acad. Sci. USA 110, 20964–20969 (2013).
Zhou, J., Lancaster, L., Donohue, J.P. & Noller, H.F. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science 345, 1188–1191 (2014).
Zhou, J., Lancaster, L., Donohue, J.P. & Noller, H.F. Crystal structures of EF-G-ribosome complexes trapped in intermediate states of translocation. Science 340, 1236086 (2013).
Dunkle, J.A. et al. Structures of the bacterial ribosome in classical and hybrid states of tRNA binding. Science 332, 981–984 (2011).
Brilot, A.F., Korostelev, A.A., Ermolenko, D.N. & Grigorieff, N. Structure of the ribosome with elongation factor G trapped in the pretranslocation state. Proc. Natl. Acad. Sci. USA 110, 20994–20999 (2013).
Savelsbergh, A., Rodnina, M.V. & Wintermeyer, W. Distinct functions of elongation factor G in ribosome recycling and translocation. RNA 15, 772–780 (2009).
Gao, Y.G. et al. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 326, 694–699 (2009).
Borg, A. et al. Fusidic acid targets elongation factor G in several stages of translocation on the bacterial ribosome. J. Biol. Chem. 290, 3440–3454 (2015).
Khade, P.K. & Joseph, S. Messenger RNA interactions in the decoding center control the rate of translocation. Nat. Struct. Mol. Biol. 18, 1300–1302 (2011).
Geggier, P. et al. Conformational sampling of aminoacyl-tRNA during selection on the bacterial ribosome. J. Mol. Biol. 399, 576–595 (2010).
Wasserman, M.R. et al. Chemically related 4,5-linked aminoglycoside antibiotics drive subunit rotation in opposite directions. Nat. Commun. 6, 7896 (2015).
Altman, R.B. et al. Enhanced photostability of cyanine fluorophores across the visible spectrum. Nat. Methods 9, 428–429 (2012).
Studer, S.M., Feinberg, J.S. & Joseph, S. Rapid kinetic analysis of EF-G-dependent mRNA translocation in the ribosome. J. Mol. Biol. 327, 369–381 (2003).
Wang, L. et al. Allosteric control of the ribosome by small-molecule antibiotics. Nat. Struct. Mol. Biol. 19, 957–963 (2012).
Wang, L., Altman, R.B. & Blanchard, S.C. Insights into the molecular determinants of EF-G catalyzed translocation. RNA 17, 2189–2200 (2011).
Pan, D., Kirillov, S.V. & Cooperman, B.S. Kinetically competent intermediates in the translocation step of protein synthesis. Mol. Cell 25, 519–529 (2007).
Peske, F., Savelsbergh, A., Katunin, V.I., Rodnina, M.V. & Wintermeyer, W. Conformational changes of the small ribosomal subunit during elongation factor G-dependent tRNA-mRNA translocation. J. Mol. Biol. 343, 1183–1194 (2004).
Feldman, M.B., Terry, D.S., Altman, R.B. & Blanchard, S.C. Aminoglycoside activity observed on single pre-translocation ribosome complexes. Nat. Chem. Biol. 6, 54–62 (2010).
Fischer, N., Konevega, A.L., Wintermeyer, W., Rodnina, M.V. & Stark, H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature 466, 329–333 (2010).
Agirrezabala, X. et al. Structural characterization of mRNA-tRNA translocation intermediates. Proc. Natl. Acad. Sci. USA 109, 6094–6099 (2012).
Adio, S. et al. Fluctuations between multiple EF-G-induced chimeric tRNA states during translocation on the ribosome. Nat. Commun. 6, 7442 (2015).
Chen, J., Petrov, A., Tsai, A., O'Leary, S.E. & Puglisi, J.D. Coordinated conformational and compositional dynamics drive ribosome translocation. Nat. Struct. Mol. Biol. 20, 718–727 (2013).
Chen, C. et al. Allosteric vs. spontaneous exit-site (E-site) tRNA dissociation early in protein synthesis. Proc. Natl. Acad. Sci. USA 108, 16980–16985 (2011).
Wilson, D.N. & Nierhaus, K.H. The E-site story: the importance of maintaining two tRNAs on the ribosome during protein synthesis. Cell. Mol. Life Sci. 63, 2725–2737 (2006).
Ferguson, A. et al. Functional dynamics within the human ribosome regulate the rate of active protein synthesis. Mol. Cell 60, 475–486 (2015).
Ermolenko, D.N. et al. The antibiotic viomycin traps the ribosome in an intermediate state of translocation. Nat. Struct. Mol. Biol. 14, 493–497 (2007).
Rodnina, M.V., Savelsbergh, A., Katunin, V.I. & Wintermeyer, W. Hydrolysis of GTP by elongation factor G drives tRNA movement on the ribosome. Nature 385, 37–41 (1997).
Savelsbergh, A. et al. An elongation factor G-induced ribosome rearrangement precedes tRNA-mRNA translocation. Mol. Cell 11, 1517–1523 (2003).
Borovinskaya, M.A., Shoji, S., Fredrick, K. & Cate, J.H. Structural basis for hygromycin B inhibition of protein biosynthesis. RNA 14, 1590–1599 (2008).
Borovinskaya, M.A., Shoji, S., Holton, J.M., Fredrick, K. & Cate, J.H. A steric block in translation caused by the antibiotic spectinomycin. ACS Chem. Biol. 2, 545–552 (2007).
Schuwirth, B.S. et al. Structures of the bacterial ribosome at 3.5 A resolution. Science 310, 827–834 (2005).
Caliskan, N., Katunin, V.I., Belardinelli, R., Peske, F. & Rodnina, M.V. Programmed -1 frameshifting by kinetic partitioning during impeded translocation. Cell 157, 1619–1631 (2014).
Katunin, V.I., Savelsbergh, A., Rodnina, M.V. & Wintermeyer, W. Coupling of GTP hydrolysis by elongation factor G to translocation and factor recycling on the ribosome. Biochemistry 41, 12806–12812 (2002).
Moazed, D. & Noller, H.F. Intermediate states in the movement of transfer RNA in the ribosome. Nature 342, 142–148 (1989).
Chen, C. et al. Single-molecule fluorescence measurements of ribosomal translocation dynamics. Mol. Cell 42, 367–377 (2011).
Walker, S.E., Shoji, S., Pan, D., Cooperman, B.S. & Fredrick, K. Role of hybrid tRNA-binding states in ribosomal translocation. Proc. Natl. Acad. Sci. USA 105, 9192–9197 (2008).
Lin, J., Gagnon, M.G., Bulkley, D. & Steitz, T.A. Conformational changes of elongation factor G on the ribosome during tRNA translocation. Cell 160, 219–227 (2015).
Liu, T. et al. Direct measurement of the mechanical work during translocation by the ribosome. eLife 3, e03406 (2014).
Ramrath, D.J. et al. The complex of tmRNA–SmpB and EF-G on translocating ribosomes. Nature 485, 526–529 (2012).
Márquez, V., Wilson, D.N., Tate, W.P., Triana-Alonso, F. & Nierhaus, K.H. Maintaining the ribosomal reading frame: the influence of the E site during translational regulation of release factor 2. Cell 118, 45–55 (2004).
Munro, J.B., Altman, R.B., Tung, C.S., Sanbonmatsu, K.Y. & Blanchard, S.C. A fast dynamic mode of the EF-G-bound ribosome. EMBO J. 29, 770–781 (2010).
Dave, R., Terry, D.S., Munro, J.B. & Blanchard, S.C. Mitigating unwanted photophysical processes for improved single-molecule fluorescence imaging. Biophys. J. 96, 2371–2381 (2009).
Aitken, C.E., Marshall, R.A. & Puglisi, J.D. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys. J. 94, 1826–1835 (2008).
Acknowledgements
We thank R. Green (Johns Hopkins University) for providing the S13-knockout strain and P. Schultz (Scripps Research Institute) for providing the L5-knockout strain. We also thank D. Terry of the Blanchard laboratory for assistance with three-color FRET data acquisition and analysis. This work was supported by the US National Institutes of Health (2R01GM079238 and 5R01GM098859 to S.C.B.).
Author information
Authors and Affiliations
Contributions
M.R.W. prepared dye-labeled ribosomes and EF-G. R.B.A. and M.R.W. prepared dye-labeled tRNAs. J.L.A. and M.R.W. performed the smFRET imaging. J.L.A. analyzed the smFRET results. J.L.A. and M.R.W. made the figures. S.C.B., M.R.W. and J.L.A. designed the study. All authors discussed the results and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.C.B. and R.B.A. have an equity interest in Lumidyne Technologies.
Integrated supplementary information
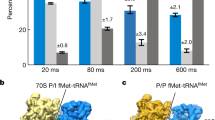
Supplementary Figure 1 Kinetic analysis of the S13-L1 FRET translocation signal.
(a,b) Representative traces of (a) initiator and (b) elongator complexes imaged during translocation. The EF-G injection, low-FRET arrival (Δtlow; POST formation), (productive) intermediate-FRET arrival (Δtarr) and intermediate-FRET dwell (Δtint) times are indicated. (c) Cumulative distributions of the waiting times to irreversible transitions from the high-FRET (rotated, PRE) state to the stable low-FRET (unrotated, POST) state. The distributions are shown for 1 mM GTP and varying concentrations of EF-G (0.25 μM EF-G, squares; 1 μM EF-G, circles; 10 μM EF-G, triangles; 10 μM EF-G with 2mM GDPNP, inverted triangles). The EF-G(GTP) distributions were fit by double exponentials (black curves). The EF-G(GDPNP) distribution was fit to a single exponential (red curve). (d–f) Waiting time distributions acquired across a range of EF-G concentrations were fit to double (Δtlow and Δtarr) or single exponentials (Δtint). The fast component rates of (d) low-FRET and (e) intermediate-FRET arrival displayed an EF-G concentration dependence that was well described by hyperbolic functions (blue – initiator; red – elongator), whereas the slow component rates did not vary with EF-G concentration (dashed lines). (f) The rates of exit from the intermediate-FRET state into the low-FRET, POST state were independent of EF-G concentration. Error bars represent standard deviations from 3 independent data sets. Fast component percentages from the double exponential fits as well as hyperbolic and linear fit parameters are indicated.
Supplementary Figure 2 Kinetic analysis of the tRNA-tRNA FRET translocation signal.
(a,b) Representative traces of (a) initiator and (b) elongator complexes imaged during translocation. The EF-G injection, (productive) high-FRET state arrival (ΔtHF arr), high-FRET state dwell (ΔtHF) and FRET loss (ΔtFRET loss; E-site tRNA departure) times are indicated. (c–e) Waiting time distributions acquired across a range of EF-G concentrations were fit to double (ΔtHF arr and ΔtFRET loss) or single exponentials (ΔtHF). (c) The fast component rates of high-FRET arrival displayed an EF-G concentration dependence that was well described by hyperbolic functions (blue – initiator; red – elongator), whereas the slow component rates did not vary with EF-G concentration (dashed lines). (d) The rates corresponding to the high-FRET state lifetime were independent of EF-G concentration. (e) The fast component rates of high-FRET loss displayed an EF-G concentration dependence that was well described by hyperbolic functions (blue – initiator; red – elongator), whereas the slow component rates did not vary with EF-G concentration (dashed lines). Error bars represent standard deviations from 3 independent data sets. Fast component percentages from the double exponential fits as well as hyperbolic and linear fit parameters are indicated.
Supplementary Figure 3 Kinetic analysis of the S13–A-site tRNA FRET translocation signal.
(a,b) Representative traces of (a) initiator and (b) elongator complexes imaged during translocation. The EF-G injection and waiting times to FRET rise (Δtrise; POST formation) are indicated. (c) Waiting time distributions acquired across a range of EF-G concentrations were fit to double exponentials (Δtrise). The fast component rates of FRET rise displayed an EF-G concentration dependence that was well described by hyperbolic functions (blue – initiator; red – elongator), whereas the slow component rates did not vary with EF-G concentration (dashed lines). Error bars represent standard deviations from 3 independent data sets. Fast component percentages from the double exponential fits as well as hyperbolic and linear fit parameters are indicated.
Supplementary Figure 4 Kinetic analysis of the S13-L5 FRET translocation signal.
(a,b) Representative traces of (a) initiator and (b) elongator complexes imaged during translocation. The EF-G injection and high-FRET arrival (Δtrise; POST formation) times are indicated. (c) Waiting time distributions acquired across a range of EF-G concentrations were fit to double exponentials (Δthigh). The fast component rates of high-FRET arrival displayed an EF-G concentration dependence that was well described by hyperbolic functions (blue – initiator; red – elongator), whereas the slow component rates did not vary with EF-G concentration (dashed lines). Error bars represent standard deviations from 3 independent data sets. Fast component percentages from the double exponential fits as well as hyperbolic and linear fit parameters are indicated.
Supplementary Figure 5 Antibiotic effects on early events in translocation.
The effects of the translocation inhibitors hygromycin B (HygB), viomycin (Vio) and spectinomycin (Spc) on the action of saturating EF-G(GTP) (10 μM EF-G; 1 mM GTP) is displayed for elongator complexes labeled on S13-L1, tRNA-tRNA, S13-A-site tRNA and S13-L5. To more clearly demonstrate drug activity on EF-G-bound complexes, traces initially in the FRET states corresponding to the rotated/hybrid conformation were selected. (a) HygB (400 μM) inhibits formation of the translocation intermediate in both the S13-L1 and S13-L5 signals. The dynamic FRET increase observed in the tRNA-tRNA signal, and the slight FRET increase observed in the S13-A-site tRNA signal suggest that hygromycin B allows EF-G to stabilize the A/P hybrid state, but inhibits formation of the head-swiveled, fully tRNA-compacted intermediate observed during uninhibited translocation. (b) Viomycin (200 μM) inhibits all transitions from the rotated, hybrid tRNA state to the intermediate or POST conformations. (c) Spectinomycin (3 mM) blocks the formation of the intermediate conformation identified in this study. However, it stabilizes what appears to be another intermediate conformation (INT1; Pan, D. et al., Mol Cell. 25, 519–529, 2007). The S13-L1 and S13-L5 panels suggest that Spc only allows a detectable, but limited amount of head swivel, while the tRNA-tRNA and S13-A-site tRNA signals suggest that the drug does not inhibit tRNA motions (compaction).
Supplementary Figure 6 Postsynchronized population FRET histograms of the translocation reaction coordinate from the perspective of motion.
Population FRET histograms showing the response of translocating elongator PRE complexes in response to the addition of EF-G(GTP) (10 μM; 1 mM) when site-specifically labeled as described in Figures 2 and 3 at (a) ribosomal proteins S13 and L1, (b) deacyl- and peptidyl-tRNA and (c) ribosomal protein S13 and peptidyl-tRNA, where each data set was post-synchronized to the final FRET state observed during translocation.
Supplementary Figure 7 Exaggerated motions of the small-subunit head domain are observed from two distinct structural perspectives.
(a) Schematic of the translocation reaction coordinate monitored by an smFRET labeling strategy that reports on P-site tRNA (orange) movement relative to the small subunit head domain protein S13 (light grey). The sites of labeling of donor and acceptor fluorophores are indicated with green and red circles, respectively. FRET values differ between initiator and elongator complexes due to different sites of P-site tRNA labeling (Online Methods). Traces initially in the FRET states corresponding to the rotated/hybrid conformation were selected. (b) Following EF-G(GTP) delivery, complexes transition from high-FRET (0.59 ± 0.07 – initiator, not shown; 0.80 ± 0.06 – elongator, shown) to lower FRET states, followed by FRET loss (E-site tRNA departure). (c) Fusidic acid (FA) stabilizes an intermediate-FRET state (0.50 ± 0.08 – initiator, not shown; 0.64 ± 0.08 – elongator, shown) and is followed by FRET loss. Inspection of individual S13-P-site tRNA traces revealed a low-FRET ‘dip’ from the FA-stabilized intermediate prior to FRET loss. To more clearly demonstrate the order and timing of this apparent additional FRET state, population histograms of (d) initiator and (e) elongator traces were post-synchronized to intermediate-FRET following the appearance of the FRET dip. Representative traces demonstrate fluctuations between the intermediate-FRET (FA-stabilized, INT2) and low-FRET states (INT3; ~0.40 and ~0.55 for initiator and elongator, respectively) prior to FRET loss. As highlighted in Figure 6, similar results are observed with the S13-L5 (f) initiator and (g) elongator complexes. Dashed lines specify the FRET state assignments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–5 (PDF 2034 kb)
Source data
Rights and permissions
About this article
Cite this article
Wasserman, M., Alejo, J., Altman, R. et al. Multiperspective smFRET reveals rate-determining late intermediates of ribosomal translocation. Nat Struct Mol Biol 23, 333–341 (2016). https://doi.org/10.1038/nsmb.3177
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3177
This article is cited by
-
Visualizing translation dynamics at atomic detail inside a bacterial cell
Nature (2022)
-
Altered tRNA dynamics during translocation on slippery mRNA as determinant of spontaneous ribosome frameshifting
Nature Communications (2022)
-
Getting to the bottom of lncRNA mechanism: structure–function relationships
Mammalian Genome (2022)
-
Time-resolved cryo-EM visualizes ribosomal translocation with EF-G and GTP
Nature Communications (2021)
-
Synthesis, structural characterization, DFT, kinetics and mechanism of oxidation of bromothymol blue: application to textile industrial wastewater treatment
Chemical Papers (2021)