Abstract
Aberrant activation of the EGF receptor (EGFR) contributes to many human cancers by activating the Ras-MAPK pathway and other pathways. EGFR signaling is augmented by Src-family kinases, but the mechanism is poorly understood. Here, we show that human EGFR preferentially phosphorylates peptide substrates that are primed by a prior phosphorylation. Using peptides based on the sequence of the adaptor protein Shc1, we show that Src mediates the priming phosphorylation, thus promoting subsequent phosphorylation by EGFR. Importantly, the doubly phosphorylated Shc1 peptide binds more tightly than singly phosphorylated peptide to the Ras activator Grb2; this binding is a key step in activating the Ras-MAPK pathway. Finally, a crystal structure of EGFR in complex with a primed Shc1 peptide reveals the structural basis for EGFR substrate specificity. These results provide a molecular explanation for the integration of Src and EGFR signaling with downstream effectors such as Ras.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yarden, Y. & Sliwkowski, M.X. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 (2001).
Zhang, X., Gureasko, J., Shen, K., Cole, P.A. & Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125, 1137–1149 (2006).
Ciardiello, F. & Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 358, 1160–1174 (2008).
Wheeler, D.L., Dunn, E.F. & Harari, P.M. Understanding resistance to EGFR inhibitors: impact on future treatment strategies. Nat. Rev. Clin. Oncol. 7, 493–507 (2010).
Bromann, P.A., Korkaya, H. & Courtneidge, S.A. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 23, 7957–7968 (2004).
Tice, D.A., Biscardi, J.S., Nickles, A.L. & Parsons, S.J. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA 96, 1415–1420 (1999).
Biscardi, J.S. et al. c-Src mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274, 8335–8343 (1999).
Moro, L. et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 277, 9405–9414 (2002).
Boerner, J.L., Biscardi, J.S., Silva, C.M. & Parsons, S.J. Transactivating agonists of the EGF receptor require Tyr845 phosphorylation for induction of DNA synthesis. Mol. Carcinog. 44, 262–273 (2005).
Biscardi, J.S., Belsches, A.P. & Parsons, S.J. Characterization of human epidermal growth factor receptor and c-Src in human breast tumor cells. Mol. Carcinog. 21, 261–272 (1998).
Maa, M.C., Leu, T.H., McCarley, D.J., Schatzman, R.C. & Parsons, S.J. Potentiation of EGF receptor-mediated oncogenesis by c-SRC: implications for the etiology of multiple human cancers. Proc. Natl. Acad. Sci. USA 92, 6981–6985 (1995).
Dimri, M. et al. Modeling breast cancer-associated c-SRC and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer Res. 67, 4164–4172 (2007).
Fu, Y.-N. et al. EGFR mutants found in non-small cell lung cancer show different levels of sensitivity to suppression of Src: implications in targeting therapy. Oncogene 27, 957–965 (2008).
Chung, B.M. et al. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene 28, 1821–1832 (2009).
Mueller, K.L., Hunter, L.A., Ethier, S.P. & Boerner, J.L. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 68, 3314–3322 (2008).
Wheeler, D.L. et al. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol. Ther. 8, 696–703 (2009).
Filosto, S., Baston, D.S., Chung, S., Becker, C.R. & Goldkorn, T. Src mediates cigarette smoke-induced resistance to tyrosine kinase inhibitors in NSCLC cells. Mol. Cancer Ther. 12, 1579–1590 (2013).
Nolen, B., Taylor, S. & Ghosh, G. Regulation of protein kinases: controlling activity through activation segment conformation. Mol. Cell 15, 661–675 (2004).
Gotoh, N., Tojo, A., Hino, M., Yazaki, Y. & Shibuya, M. A highly conserved tyrosine residue at codon 845 within the kinase domain is not required for the transforming activity of human epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 186, 768–774 (1992).
Mueller, K.L., Powell, K., Madden, J.M., Eblen, S.T. & Boerner, J.L. EGFR tyrosine 845 phosphorylation-dependent proliferation and transformation of breast cancer cells require activation of p38 MAPK. Transl. Oncol. 5, 327–334 (2012).
Hutti, J.E. et al. A rapid method for determining protein kinase phosphorylation specificity. Nat. Methods 1, 27–29 (2004).
Songyang, Z. et al. Catalytic specificity of protein tyrosine kinases is critical for selective signaling. Nature 373, 536–539 (1995).
Chan, S.K., Gullick, W.J. & Hill, M.E. Mutations of the epidermal growth factor receptor in non-small cell lung cancer: search and destroy. Eur. J. Cancer 42, 17–23 (2006).
Hornbeck, P.V. et al. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in human and mouse. Nucleic Acids Res. 40, D261–D270 (2012).
Sato, K. et al. Tyrosine residues 239 and 240 of Shc are phosphatidylinositol 4,5-bisphosphate-dependent phosphorylation sites by c-Src. Biochem. Biophys. Res. Commun. 240, 399–404 (1997).
Wills, M.K.B. & Jones, N. Teaching an old dogma new tricks: twenty years of Shc adaptor signaling. Biochem. J. 447, 1–16 (2012).
van der Geer, P., Wiley, S., Gish, G.D. & Pawson, T. The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr. Biol. 6, 1435–1444 (1996).
Songyang, Z. et al. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol. 14, 2777–2785 (1994).
Ngo, J.C.K. et al. A sliding docking interaction is essential for sequential and processive phosphorylation of an SR protein by SRPK1. Mol. Cell 29, 563–576 (2008).
Luttrell, L.M. et al. Role of c-Src tyrosine kinase in G protein-coupled receptor and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 271, 19443–19450 (1996).
Nioche, P. et al. Crystal structures of the SH2 domain of Grb2: highlight on the binding of a new high-affinity inhibitor. J. Mol. Biol. 315, 1167–1177 (2002).
Hubbard, S.R. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 16, 5572–5581 (1997).
Favelyukis, S., Till, J.H., Hubbard, S.R. & Miller, W.T. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat. Struct. Biol. 8, 1058–1063 (2001).
Levinson, N.M. et al. A Src-like inactive conformation in the abl tyrosine kinase domain. PLoS Biol. 4, e144 (2006).
Guo, A. et al. Signaling networks assembled by oncogenic EGFR and c-MET. Proc. Natl. Acad. Sci. USA 105, 692–697 (2008).
Park, E. et al. Structure and mechanism of activity-based inhibition of the EGF receptor by Mig6. Nat. Struct. Mol. Biol. 22, 703–711 (2015).
Ogura, K. et al. Solution structure of the SH2 domain of Grb2 complexed with the Shc-derived phosphotyrosine-containing peptide. J. Mol. Biol. 289, 439–445 (1999).
Lubman, O.Y. & Waksman, G. Structural and thermodynamic basis for the interaction of the Src SH2 domain with the activated form of the PDGF beta-receptor. J. Mol. Biol. 328, 655–668 (2003).
Chen, C.H., Martin, V.A., Gorenstein, N.M., Geahlen, R.L. & Post, C.B. Two closely spaced tyrosines regulate NFAT signaling in B cells via Syk association with Vav. Mol. Cell. Biol. 31, 2984–2996 (2011).
Groesch, T.D., Zhou, F., Mattila, S., Geahlen, R.L. & Post, C.B. Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J. Mol. Biol. 356, 1222–1236 (2006).
Weber, T., Schaffhausen, B., Liu, Y. & Gunther, U.L. NMR structure of the N-SH2 of the p85 subunit of phosphoinositide 3-kinase complexed to a doubly phosphorylated peptide reveals a second phosphotyrosine binding site. Biochemistry 39, 15860–15869 (2000).
Fiol, C.J., Mahrenholz, A.M., Wang, Y., Roeske, R.W. & Roach, P.J. Formation of protein kinase recognition sites by covalent modification of the substrate: molecular mechanism for the synergistic action of casein kinase 2 and glycogen synthase kinase 3. J. Biol. Chem. 262, 14042–14048 (1987).
Flotow, H. et al. Phosphate groups as substrate determinants for casein kinase 1 action. J. Biol. Chem. 265, 14264–14269 (1990).
Olsen, J.V. et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 (2006).
Schweiger, R. & Linial, M. Cooperativity within proximal phosphorylation sites is revealed from large-scale proteomics data. Biol. Direct 5, 6 (2010).
Hutti, J.E. et al. IκB kinase β phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-κB pathway. Mol. Cell. Biol. 27, 7451–7461 (2007).
Davis, T.L. et al. Structural recognition of an optimized substrate for the ephrin family of receptor tyrosine kinases. FEBS J. 276, 4395–4404 (2009).
Bouskila, M. et al. TTBK2 kinase substrate specificity and the impact of spinocerebellar-ataxia-causing mutations on expression, activity, localization, and development. Biochem. J. 437, 157–167 (2011).
Chen, S. et al. Tyrosine kinase BMX phosphorylates phosphotyrosine-primed motif mediating the activation of multiple receptor tyrosine kinases. Sci. Signal. 6, ra40 (2013).
Yun, C.-H. et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 11, 217–227 (2007).
Xu, W., Harrison, S.C. & Eck, M.J. Three-dimensional structure of the tyrosine kinase c-SRC. Nature 385, 595–602 (1997).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Acknowledgements
We thank S. Breitkopf and M. Yuan for help with MS experiments and Y. Zheng for help with HPLC experiments. We thank B. Murray for critical reading of the manuscript. This work was partially supported by US National Institutes of Health grants 2P01CA120964 (J.M.A.) and S10OD010612 (J.M.A.).
Author information
Authors and Affiliations
Contributions
M.J.B. designed and conducted all biochemical, enzymatic and cell-based experiments. C.Y. determined all crystal structures. C.A.G. and J.L.J., together with M.J.B., performed peptide library experiments. J.M.A. executed mass spectrometry–based experiments. M.J.E. conceived and supervised structural experiments. I.A. and A.J.C. conceived and supervised cell-based experiments. L.C.C., together with M.J.B., conceived the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 EGFR and Src peptide library assays using specific inhibitors.
(a) Phosphorylation of the pY+1 peptide library by EGFR WT or EGFR L858R in the presence of increasing concentrations of gefitinib. Activity is graphed as percent of control (no gefitinib). (b) Phosphorylation of the I-1, G+1, pY+1, and Y+3 peptide libraries by Src in the presence of increasing concentrations of dasatinib. Activity is graphed as percent of control (no dasatinib).
Supplementary Figure 2 EGFR activity toward peptides based on the sequence surrounding MET Tyr1234.
Peptides were phosphorylated in vitro with recombinant EGFR (MYDKEYYSVHNKK = Y-Y; MYDKEYpYSVHNKK = Y-pY). Error bars, s.d. (n=3 technical replicates).
Supplementary Figure 3 Specificity of phospho-Shc pY239/240–specific antibody.
Serial dilutions of peptides corresponding to the sequence surrounding Tyr239 of Shc1 were spotted on nitrocellulose and probed with a phospho-Shc pY239/240 antibody (Cell Signaling Technology Cat #2434; PDHQYYNDAKKK = Y-Y; PDHQYpYNDAKKK = Y-pY; PDHQpYYNDAKKK = pY-Y; PDHQpYpYNDAKKK = pY-pY). 40nmol corresponds to 2μL of a 20mM stock solution of each peptide (20mM is the solubility limit of the peptides in Tris buffer).
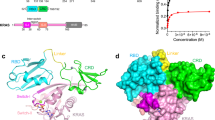
Supplementary Figure 4 Crystal structures of EGFR in complex with peptides.
(a) (b) Electron density of peptides in complex with EGFR L858R kinase domain. Fo-Fc difference omit maps (in which the peptides were omitted) for the optimal peptide (a) or the primed Shc1 peptide (b) contoured at 3σ. (c) Interactions between the EGFR L858R kinase domain and the optimal substrate peptide (DEEDYpYEIP). Hydrogen bonds are indicated with dashed lines.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1 and 2 (PDF 1675 kb)
Supplementary Data Set 1
Original western blot images (PDF 948 kb)
Rights and permissions
About this article
Cite this article
Begley, M., Yun, Ch., Gewinner, C. et al. EGF-receptor specificity for phosphotyrosine-primed substrates provides signal integration with Src. Nat Struct Mol Biol 22, 983–990 (2015). https://doi.org/10.1038/nsmb.3117
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3117
This article is cited by
-
Spatial-proteomics reveals phospho-signaling dynamics at subcellular resolution
Nature Communications (2021)
-
Overcoming EGFRG724S-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors
Nature Communications (2018)
-
Spatial cycles mediated by UNC119 solubilisation maintain Src family kinases plasma membrane localisation
Nature Communications (2017)
-
Biosensor of alkaline phosphatase based on non-fluorescent FRET of Eu3+-doped oxide nanoparticles and phosphorylated peptide labeled with cyanine dye
Analytical and Bioanalytical Chemistry (2017)
-
Src defines a new pool of EGFR substrates
Nature Structural & Molecular Biology (2015)