Abstract
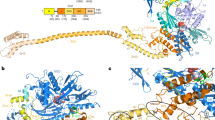
Ribosome synthesis involves dynamic association of ribosome-biogenesis factors with evolving preribosomal particles. Rio2 is an atypical protein kinase required for pre-40S subunit maturation. We report the crystal structure of eukaryotic Rio2–ATP–Mg2+ complex. The active site contains ADP-Mg2+ and a phosphoaspartate intermediate typically found in Na+, K+ and Ca2+ ATPases but not protein kinases. Consistent with this finding, ctRio2 exhibits a robust ATPase activity in vitro. In vivo, Rio2 docks on the ribosome, with its active site occluded and its flexible loop positioned to interact with the pre-40S subunit. Moreover, Rio2 catalytic activity is required for its dissociation from the ribosome, a necessary step in pre-40S maturation. We propose that phosphoryl transfer from ATP to Asp257 in Rio2's active site and subsequent hydrolysis of the aspartylphosphate could be a trigger to power late cytoplasmic 40S subunit biogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
05 December 2012
In the version of this supplementary file originally posted online, the labels for the chemicals shown in Supplementary Figure 5d contained errors. The errors have been corrected in this file 5 December 2012.
References
Geerlings, T.H., Faber, A.W., Bister, M.D., Vos, J.C. & Raue, H.A. Rio2p, an evolutionarily conserved, low abundant protein kinase essential for processing of 20 S pre-rRNA in Saccharomyces cerevisiae. J. Biol. Chem. 278, 22537–22545 (2003).
Vanrobays, E., Gelugne, J.P., Gleizes, P.E. & Caizergues-Ferrer, M. Late cytoplasmic maturation of the small ribosomal subunit requires RIO proteins in Saccharomyces cerevisiae. Mol. Cell Biol. 23, 2083–2095 (2003).
Schäfer, T., Strauss, D., Petfalski, E., Tollervey, D. & Hurt, E. The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes. EMBO J. 22, 1370–1380 (2003).
Henras, A.K. et al. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol. Life Sci. 65, 2334–2359 (2008).
Fromont-Racine, M., Senger, B., Saveanu, C. & Fasiolo, F. Ribosome assembly in eukaryotes. Gene 313, 17–42 (2003).
Zemp, I. et al. Distinct cytoplasmic maturation steps of 40S ribosomal subunit precursors require hRio2. J. Cell Biol. 185, 1167–1180 (2009).
Granneman, S., Petfalski, E., Swiatkowska, A. & Tollervey, D. Cracking pre-40S ribosomal subunit structure by systematic analyses of RNA-protein cross-linking. EMBO J. 29, 2026–2036 (2010).
Strunk, B.S. et al. Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates. Science 333, 1449–1453 (2011).
LaRonde-LeBlanc, N., Guszczynski, T., Copeland, T. & Wlodawer, A. Autophosphorylation of Archaeoglobus fulgidus Rio2 and crystal structures of its nucleotide-metal ion complexes. FEBS J. 272, 2800–2810 (2005).
LaRonde-LeBlanc, N. & Wlodawer, A. Crystal structure of A. fulgidus Rio2 defines a new family of serine protein kinases. Structure 12, 1585–1594 (2004).
Amlacher, S. et al. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell 146, 277–289 (2011).
Post, R.L. & Kume, S. Evidence for an aspartyl phosphate residue at the active site of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 248, 6993–7000 (1973).
Kühlbrandt, W. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 5, 282–295 (2004).
Hanks, S.K., Quinn, A.M. & Hunter, T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241, 42–52 (1988).
Taylor, S.S. & Kornev, A.P. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem. Sci. 36, 65–77 (2011).
Sanders, D.A., Gillece-Castro, B.L., Stock, A.M., Burlingame, A.L. & Koshland, D.E. Jr. Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J. Biol. Chem. 264, 21770–21778 (1989).
Collet, J.F., Stroobant, V., Pirard, M., Delpierre, G. & Van Schaftingen, E. A new class of phosphotransferases phosphorylated on an aspartate residue in an amino-terminal DXDX(T/V) motif. J. Biol. Chem. 273, 14107–14112 (1998).
Zheng, J. et al. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochemistry 32, 2154–2161 (1993).
Parang, K. & Cole, P.A. Designing bisubstrate analog inhibitors for protein kinases. Pharmacol. Ther. 93, 145–157 (2002).
Schäfer, T. et al. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature 441, 651–655 (2006).
Aaronson, R.P. & Blobel, G. On the attachment of the nuclear pore complex. J. Cell Biol. 62, 746–754 (1974).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Trabuco, L.G., Villa, E., Mitra, K., Frank, J. & Schulten, K. Flexible fitting of atomic structures into electron microscopy maps using molecular dynamics. Structure 16, 673–683 (2008).
Bishop, A.C., Buzko, O. & Shokat, K.M. Magic bullets for protein kinases. Trends Cell Biol. 11, 167–172 (2001).
Palmgren, M.G. & Nissen, P. P-type ATPases. Annu. Rev. Biophys. 40, 243–266 (2011).
Ye, Q., Crawley, S.W., Yang, Y., Cote, G.P. & Jia, Z. Crystal structure of the alpha-kinase domain of Dictyostelium myosin heavy chain kinase A. Sci. Signal. 3, ra17 (2010).
Xu, W., Doshi, A., Lei, M., Eck, M.J. & Harrison, S.C. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell 3, 629–638 (1999).
Nagar, B. et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 112, 859–871 (2003).
Malakhova, M. et al. Structural basis for activation of the autoinhibitory C-terminal kinase domain of p90 RSK2. Nat. Struct. Mol. Biol. 15, 112–113 (2008).
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334, 1524–1529 (2011).
Janke, C. et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 (2004).
Longtine, M.S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Puig, O. et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24, 218–229 (2001).
Peluso, P., Shan, S.O., Nock, S., Herschlag, D. & Walter, P. Role of SRP RNA in the GTPase cycles of Ffh and FtsY. Biochemistry 40, 15224–15233 (2001).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Delano, W. The Pymol molecular graphics system (Delano Scientific, 2002).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph 14, 33–38, 27–28 (1996).
Acknowledgements
We thank K. Shokat (Howard Hughes Medical Institute and Department of Cellular and Molecular Pharmacology, University of California, San Francisco, San Francisco California, USA) for providing the ATP analog compounds 3-MB-PP1 and 1-NA-PP1; K. Karbstein (Scripps Research Institute, Jupiter, Florida, USA) for providing anti-Tsr1 antibody; S. Amlacher for providing C. thermophilum cDNA and E. Thomson and S. Griesel for providing ctHrr25 expression vector (Biochemistry Center, University of Heidelberg, Heidelberg, Germany); J. Lechner and his team for mass spectrometry, G. Lorimer (Department of Chemistry and Biochemistry, University of Maryland, College Park, Maryland, USA) for assistance and advice in steady-state kinetic measurements, G. Manikas for assistance with single-turnover and ATP-binding assays and M. Gnädig for her excellent technical assistance. Data collection was conducted at the Advanced Photon Source on the Northeastern Collaborative Access Team beamlines, supported by grants from the US National Center for Research Resources (5P41RR015301-10) and the US National Institute of General Medical Sciences (8 P41 GM103403-10) from the US National Institutes of Health. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under contract no. DE-AC02-06CH11357. This work was funded by the postdoctoral fellowship from the Medical Faculty of the University of Heidelberg to S.F.-C., the German Research Council (DFG Hu363/10-4) to E.H. and US National Institutes of Health National Cancer Institute grant (K22CA123152) to N.L.-L.
Author information
Authors and Affiliations
Contributions
S.F.-C., T.S., N.L.-L. and E.H. conceived of the experiments. S.F.-C., T.S. and A.-M.W. constructed all plasmids and yeast strains and carried out all yeast genetic experiments. S.F.-C. performed all the sucrose-gradient analyses, tandem-affinity purifications of yeast proteins and single-turnover and nucleotide-binding analyses. T.S. performed in vitro phosphorylation experiments on purified pre-40S. S.F.-C. and A.-M.W. performed the biochemical characterization of ctRio2. V.S. and E.C. optimized and performed protein purification and identified and refined crystallization conditions. V.S. and N.L.-L. determined the crystal structures. M.D. performed hydroxylamine phosphate release assays and steady-state rate determination using purified protein provided by H.L. N.L.-L. performed ctRio2-40S docking analysis. E.H. and N.L.-L. supervised the work; S.F.-C., N.L.-L. and E.H. wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–2 and Supplementary Note (PDF 6835 kb)
Rights and permissions
About this article
Cite this article
Ferreira-Cerca, S., Sagar, V., Schäfer, T. et al. ATPase-dependent role of the atypical kinase Rio2 on the evolving pre-40S ribosomal subunit. Nat Struct Mol Biol 19, 1316–1323 (2012). https://doi.org/10.1038/nsmb.2403
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2403
This article is cited by
-
Identification of RIOK2 as a master regulator of human blood cell development
Nature Immunology (2022)
-
Blockade of IL-22 signaling reverses erythroid dysfunction in stress-induced anemias
Nature Immunology (2021)
-
Crystal structure of the human PRPK–TPRKB complex
Communications Biology (2021)
-
Characterization of a toxin-antitoxin system in Mycobacterium tuberculosis suggests neutralization by phosphorylation as the antitoxicity mechanism
Communications Biology (2020)
-
Noncanonical CTD kinases regulate RNA polymerase II in a gene-class-specific manner
Nature Chemical Biology (2019)