Abstract
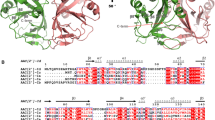
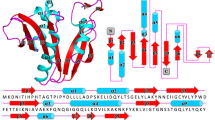
AAC(2′)-Ic catalyzes the coenzyme A (CoA)-dependent acetylation of the 2′ hydroxyl or amino group of a broad spectrum of aminoglycosides. The crystal structure of the AAC(2′)-Ic from Mycobacterium tuberculosis has been determined in the apo enzyme form and in ternary complexes with CoA and either tobramycin, kanamycin A or ribostamycin, representing the first structures of an aminoglycoside acetyltransferase bound to a drug. The overall fold of AAC(2′)-Ic places it in the GCN5-related N-acetyltransferase (GNAT) superfamily. Although the physiological function of AAC(2′)-Ic is uncertain, a structural analysis of these high-affinity aminoglycoside complexes suggests that the enzyme may acetylate a key biosynthetic intermediate of mycothiol, the major reducing agent in mycobacteria, and participate in the regulation of cellular redox potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shaw, K.J., Rather, P.N., Hare, R.S. & Miller, G.H. Microbiol. Rev. 57, 138–163 (1993).
Macinga, D.R. & Rather, P.N. Front. Biosci. 4, 132–140 (1999).
Rather, P.N., Orosz, E., Shaw, K.J., Hare, R. & Miller, G. J. Bacteriol. 175, 6492–6498 (1993).
Ainsa, J.A. et al. Mol. Microbiol. 24, 431–441 (1997).
Hegde, S.S., Javid-Majd, F. & Blanchard, J.S. J. Biol. Chem. 276, 45876–45881 (2001).
Dyda, F., Klein, D.C. & Hickman, A.B. Annu. Rev. Biophys. Biomol. Struct. 29, 81–103 (2000).
Peneff, C., Mengin-Lecreulx, D. & Bourne, Y. J. Biol. Chem. 276, 16328–16334 (2001).
Hickman, A.B., Klein, D.C. & Dyda, F. Mol. Cell 3, 23–32 (1999).
Hickman, A.B., Namboodiri, M.A., Klein, D.C. & Dyda, F. Cell 97, 361–369 (1999).
Dutnall, R.N., Tafrov, S.T., Sternglanz, R. & Ramakrishnan, V. Cell 94, 427–438 (1998).
Angus-Hill, M.L., Dutnall, R.N., Tafrov, S.T., Sternglanz, R. & Ramakrishnan, V. J. Mol. Biol. 294, 1311–1325 (1999).
Clements, A. et al. EMBO J. 18, 3521–3532 (1999).
Trievel, R.C. et al. Proc. Natl. Acad. Sci. USA 96, 8931–8936 (1999).
Lin, Y., Fletcher, C.M., Zhou, J., Allis, C.D. & Wagner, G. Nature 400, 86–89 (1999).
Rojas, J.R. et al. Nature 401, 93–98 (1999).
Yan, Y., Barlev, N.A., Haley, R.H., Berger, S.L. & Marmorstein, R. Mol. Cell 6, 1195–1205 (2000).
Wybenga-Groot, L.E., Draker, K., Wright, G.D. & Berghuis, A.M. Struct. Fold. Des. 7, 497–507 (1999).
Wolf, E. et al. Cell 94, 439–449 (1998).
Kinoshita, K., Sadanami, K., Kidera, A. & Go, N. Protein Eng. 12, 11–14 (1999).
Marmorstein, R. J. Mol. Biol. 311, 433–444 (2001).
Bhatnagar, R.S., Futterer, K., Waksman, G. & Gordon, J.I. Biochim. Biophys. Acta 1441, 162–172 (1999).
Payie, K.G. & Clarke, A.J. J. Bacteriol. 179, 4106–4114 (1997).
Newton, G.L. et al. J. Bacteriol. 178, 1990–1995 (1996).
Macinga, D.R., Cook, G.M., Poole, R.K. & Rather, P.N. J. Bacteriol. 180, 128–135 (1998).
Macinga, D.R., Paradise, M.R., Parojcic, M.M. & Rather, P.N. Antimicrob. Agents Chemother. 43, 1769–1772 (1999).
Newton, G.L., Av-Gay, Y. & Fahey, R.C. J. Bacteriol. 182, 6958–6963 (2000).
Anderberg, S.J., Newton, G.L. & Fahey, R.C. J. Biol. Chem. 273, 30391–30397 (1998).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Levitt, D.G. Acta Crystallogr. D 57, 1013–1019 (2001).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Esnouf, R.M. J. Mol. Graph. 15, 132–134 (1997).
Merritt, E.A. & Bacon, D.J. Methods Enzymol. 277, 505–524 (1997).
Lu, G. J. Appl. Crystallogr. 33, 176–183 (2000).
Acknowledgements
This work was supported by a National Institute of Allergy and Infectious Diseases grant (to J.S.B.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vetting, M., Hegde, S., Javid-Majd, F. et al. Aminoglycoside 2′-N-acetyltransferase from Mycobacterium tuberculosis in complex with coenzyme A and aminoglycoside substrates. Nat Struct Mol Biol 9, 653–658 (2002). https://doi.org/10.1038/nsb830
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb830
This article is cited by
-
Mechanistic plasticity in ApmA enables aminoglycoside promiscuity for resistance
Nature Chemical Biology (2024)
-
Novel D-glutamate catabolic pathway in marine Proteobacteria and halophilic archaea
The ISME Journal (2023)
-
Molecular basis of antibiotic self-resistance in a bee larvae pathogen
Nature Communications (2022)
-
Structural and phylogenetic analyses of resistance to next-generation aminoglycosides conferred by AAC(2′) enzymes
Scientific Reports (2021)
-
Structural and biochemical analyses of an aminoglycoside 2′-N-acetyltransferase from Mycolicibacterium smegmatis
Scientific Reports (2020)