Abstract
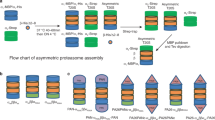
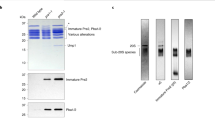
Coexpression of both subunits of the Thermoplasma proteasome in Escherichia coli yields fully assembled and proteolytically active proteasomes. Post-translational processing of the β-subunit occurs in E. coli as it does in Thermoplasma. Coexpression of the α-subunit and the βΔpro-subunit, a mutant β-subunit lacking the propeptide, also yields fully assembled and active proteasomes. This indicates that the β-propeptide is not essential for the folding and assembly of Thermoplasma proteasomes. Separately expressed α-subunits assemble into heptameric rings indistinguishable from the terminal rings of a proteasome. Mutational analysis shows that the amino terminus, which is highly conserved in all proteasomal α-type proteins, is essential for assembly. In the absence of α-subunits the β-subunits are monomeric and post-translational processing of the β-propeptide does not occur.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Goldberg, A.L. The mechanism and functions of ATP-dependent proteases in bacterial and animal cells. Eur. J. Biochem. 203, 9–23 (1992).
Tanaka, K., Tamura, T., Yoshimura, T. & Ichihara, A. Proteasomes: protein and gene structure. The New Biologist 4, 173–187 (1992).
Rivett, A.J. Proteasomes: multicatalytic proteinase complexes. Biochem. J. 291, 1–10 (1993).
Hershko, A. & Ciechanover, A. The ubiquitin system for protein degradation. A. Rev. Biochem. 61, 761–807 (1992).
Rechsteiner, M., Hoffman, L. & Dubiel, W. The multicatalytic and 26S proteases. J. biol. Chem. 268, 6065–6068 (1993).
Goldberg, A.L. & Rock, K.L. Proteolysis, proteasomes and antigen presentation. Nature 357, 375–379 (1992).
Monaco, J.J. A molecular model of MHC class-I-restricted antigen processing. Immunol. Today 13, 173–179 (1992).
Trowsdale, J. Genomic structure and function in the MHC. Trends Genet. 9, 117–122 (1993).
Schauer, T. et al. Proteasomes from Dictyostelium discoideum: characterization of structure and function. J. struct. Biol. 111, 135–147 (1993).
Hegerl, R., Pfeifer, G., Puehler, G., Dahlmann, B. & Baumeister, W. The three-dimensional structure of proteasomes from Thermoplasma acidophilum as determined by electron microscopy using random conical tilting. FEBS Letts. 283, 117–121 (1991).
Puehler, G. et al. Subunit stoichiometry and three-dimensional arrangement in proteasomes from Thermoplasma acidophilum. EMBO J. 11, 1607–1616 (1992).
Kopp, F., Dahlmann, B. & Hendil, K.B. Evidence indicating that the human proteasome is a complex dimer. J. molec. Biol. 229, 14–19 (1993).
Dahlmann, B. et al. The multicatalytic proteinase (prosome) is ubiquitous from eukaryotes to archaebacteria. FFBS Letts. 251, 125–131 (1989).
Zwickl, P., Lottspeich, F., Dahlmann, B. & Baumeister, W. Cloning and sequencing of the gene encoding the large (α-) subunit of the proteasome from Thermoplasma acidophilum. FEBS Letts. 278, 217–221 (1991).
Zwickl, P. et al. Primary structure of the Thermoplasma proteasome and its implications for the structure, function, and evolution of the multicatalytic proteinase. Biochemistry 31, 964–972 (1992).
Puehler, G., Pitzer, F., Zwickl, P. & Baumeister, W. Proteasomes: multisubunit proteinases common to Thermoplasma and Eukaryotes. System. appl. Microbiol. 16, 734–741 (1994).
Grziwa, A., Baumeister, W., Dahlmann, B. & Kopp, F. Localization of subunits in proteasomes from Thermoplasma acidophilum by immunoelectron microscopy. FEBS Letts 290, 186–190 (1991).
Zwickl, P., Lottspeich, F. & Baumeister, W. Expression of functional Thermoplasma acidophilum proteasomes in Escherichia coli. FEBS Letts 312, 157–160 (1992).
Frueh, K. et al. Alternative exon usage and processing of the major histocompatibility complex-encoded proteasome subunits. J. biol. Chem. 267, 22131–22140 (1992).
Glynne, R. et al. The major histocompatibility complex-encoded proteasome component LMP7: alternative first axons and posttranslational processing. Eur. J. Immunol. 23, 860–866 (1993).
Frentzel, S. et al. The major-histocompatibility-complex-encoded β-type proteasome subunits LMP2 and LMP7. Eur. J. Biochem. 216, 119–126 (1993).
Frentzel, S., Pesold-Hurt, B., Seelig, A. & Kloetzel, P.-M. 20S proteasomes are assembled via distinct precursor complexes. J. molec. Biol. 236, 975–981 (1994).
Martinez, C.K. & Monaco, J.J. Post-translational processing of a major histocompatibility complex-encoded proteasome subunit, LMP-2. Molec. Immunol. 30, 1177–1183 (1993).
Maurizi, M.R. Proteases and protein degradation in Escherichia coli. Experientia 48, 178–201 (1992).
Gottesman, S. & Maurizi, M.R. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol. Rev. 56, 592–621 (1992).
Baker, D., Shiau, A.K. & Agard, D.A. The role of pro regions in protein folding. Curr. Opin. Cell Biol. 5, 966–970 (1993).
Shinde, U. & Inouye, M. Intramolecular chaperones and protein folding. Trends biochem. Sci. 18, 442–446 (1993).
Seelig, A., Multhaup, G., Pesold-Hurt, B., Beyreuther, K. & Kloetzel, P.-M. Drosophila proteasomes Dm25 subunit substitutes the mouse MC3 subunit in hybrid proteasomes. J. biol. Chem. 268, 25561–25567 (1993).
Zwickl, P. et al. Electron microscopy and image analysis reveal common principles of organization in two large protein complexes: GroEL-type proteins and proteasomes. J. struct. Biol. 103, 197–203 (1990).
Horovitz, A., Bochkareva, E.S. & Girshovich, A.S. The N terminus of the molecular chaperonin GroEL is a crucial structural element for its assembly. J. biol. Chem. 268, 9957–9959 (1993).
Horovitz, A., Bochkareva, E.S., Kovalenko, O. & Girshovich, A.S. Mutation Ala2Ser destabilizes intersubunit interactions in the molecular chaperone GroEL. J. molec. Biol. 231, 58–64 (1993).
Taguchi, H., Makino, Y. & Yoshida, M. Monomeric chaperonin-60 and its 50-kda fragment possess the ability to interact with non-native proteins, to suppress aggregation, and to promote protein folding. J. biol. Chem. 269, 8529–8534 (1994).
Thornton, J.M. & Sibanda, B.L. Amino and carboxy-terminal regions in globular proteins. J. molec. Biol. 167, 443–460 (1983).
Grziwa, A., Maack, S., Puehler, G., Wiegand, G., Baumeister, W. & Jaenicke, R. Dissociation and reconstitution of the Thermoplasma proteasome. Eur. J. Biochem. 223, 1061–1067 (1994).
Akiyama, K. et al. cDNA cloning and interferon γ down-regulation of proteasomal subunits X and Y. Science 265, 1231–1234 (1994).
Belich, M.P., Glynne, R.J., Senger, G., Sheer, D. & Trowsdale, J. Proteasome components with reciprocal expression to that of the MHC-encoded LMP proteins. Curr. Biol. 4, 769–776 (1994).
Clackson, T., Guessow, D. & Jones, P.T. General application of PCR to gene cloning and manipulation. in PCR, A practical approach (eds McPherson, M.J. et al.) 187–214 (Oxford University Press, Oxford, (1991).
Lundberg, K.S. et al. High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus furiosus. Gene 108, 1–6 (1991).
Tabor, S. & Richardson, C.C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. natn. Acad. Sci. U.S.A. 82, 1074–1078 (1985).
Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zwickl, P., Kleinz, J. & Baumeister, W. Critical elements in proteasome assembly. Nat Struct Mol Biol 1, 765–770 (1994). https://doi.org/10.1038/nsb1194-765
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1194-765
This article is cited by
-
Allosteric regulation of the 20S proteasome by the Catalytic Core Regulators (CCRs) family
Nature Communications (2023)
-
Allosteric coupling between α-rings of the 20S proteasome
Nature Communications (2020)
-
A unified mechanism for proteolysis and autocatalytic activation in the 20S proteasome
Nature Communications (2016)
-
Protein Expression of Proteasome Subunits in Elderly Patients with Schizophrenia
Neuropsychopharmacology (2016)
-
Alpha-ring Independent Assembly of the 20S Proteasome
Scientific Reports (2015)