Abstract
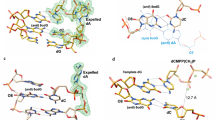
A new group of error-prone DNA polymerases overcomes the blockage posed to normal DNA replication by damaged template bases, suggesting an active site with a loose, flexible pocket that accommodates aberrant DNA structures. We have determined a 2.8 Å resolution crystal structure of the Sulfolobus solfataricus Dbh protein, a DNA translesion polymerase closely related to Escherichia coli DNA polymerase IV and human polymerase κ. A high error rate is observed for the Dbh polymerase in a range of 10−2–10−3 for all 12 base substitution mispairs. The crystal structure of Dbh reveals an overall architecture resembling other DNA polymerases but has unique features that are likely to contribute to error-prone synthesis, including −1 frameshifting mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cox, M.M. et al. Nature 404, 37–41 (2000).
Bridges, B.A. Curr. Biol. 8, 886–888 (1998).
Ohmori, H. et al. Mol. Cell 8, 7–8 (2001).
Tang, M. et al. Nature 404, 1014–1018. (2000).
Kim, S.R. et al. Proc. Natl. Acad. Sci. USA 94, 13792–13797 (1997).
Foster, P.L. Cold Spring Harbor Symposia on Quantitative Biology 65, 21–29 (2000).
McKenzie, G.J., Lee, P.L., Lombardo, M., Hastings, P.J. & Rosenberg, S.M. Mol. Cell 7, 571–579 (2001).
Johnson, R.E., Prakash, S. & Prakash, L. Proc. Natl. Acad. Sci. USA 97, 3838–3843 (2000).
Ohashi, E. et al. J. Biol. Chem. 275, 39678–39684 (2000).
Zhang, Y. et al. Nucleic Acids Res. 28, 4138–4146 (2000).
Napolitano, R., Janel-Bintz, R., Wagner, J. & Fuchs, R. P. EMBO J. 19, 6259–6265 (2000).
Zhou, B., Pata, J.D. & Steitz, T.A. Mol. Cell 8, 427–437 (2001).
Trincao, J. et al. Mol. Cell 8, 417–426 (2001).
Steitz, T.A. J. Biol. Chem. 274, 17395–17398 (1999).
Pelletier, H., Sawaya, M.R., Wolfle, W., Wilson, S.H. & Kraut, J. Biochemistry 35, 12742–12761 (1996).
Kunkel, T.A. & Soni, A. J. Biol. Chem. 263, 14784–14789 (1988).
Efrati, E., Tocco, G., Eritja, R., Wilson, S.H. & Goodman, M.F. J. Biol. Chem. 272, 2559–2569 (1997).
Doublié, S., Sawaya, M. R. & Ellenberger, T. Struct. Folding Des. 7, 31–35 (1999).
Beard, W.A. & Wilson, S.H. Mutation Res. 460, 231–244 (2000).
Kunkel, T.A. & Bebenek, K. Annu. Rev. Biochem. 69, 497–529 (2000).
Joyce, C.M. & Steitz, T.A. Annu. Rev. Biochem. 63, 777–822 (1994).
Johnson, K.A. Annu. Rev. Biochem. 62, 685–713 (1993).
Sawaya, M.R., Pelletier, H., Kumar, A., Wilson, S.H. & Kraut, J. Science 264, 1930–1935 (1994).
Doublié, S., Tabor, S., Long, A.M., Richardson, C.C. & Ellenberger, T. Nature 391, 251–258 (1998).
Wagner, J. et al. Mol. Cell 4, 281–286 (1999).
Matsuda, T., Bebenek, K., Masutani, C., Hanaoka, F. & Kunkel, T.A. Nature 404, 1011–1013 (2000).
Johnson, R.E., Washington, M.T., Prakash, S. & Prakash, L. J. Biol. Chem. 275, 7447–7450 (2000).
Creighton, S., Bloom, L.B. & Goodman, M.F. Methods Enzymol. 262, 232–256 (1995).
Otwinowski, Z. & Minor, V. Methods Enzymol. 276, 307–326 (1997).
Bailey, S. Acta Crystallogr. D 52, 30–42 (1994).
Read, R.J. Acta Crystallogr. A 42, 140–149 (1986).
Jones, T.A., Zou, J.-Y. & Cowan, S.W. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Kissinger, C.R., Gehlhaar, D.K. & Fogel, D.B. Acta Crystallogr. D 55, 484–491 (1999).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Acknowledgements
This work was supported by research grants from the U.S. Department of Energy (to T.E.) and the National Insitutes of Health (to M.F.G.). L.F.S and E.A.T. are supported by postdoctoral fellowships from the National Institutes of Health. We thank the staff of the Harvard/Armenise Structural Biology Center (Harvard Medical School) and beamlines X-12C and X-25 of the Macromolecular Crystallography Research Resource at the National Synchrotron Light Source (Upton, NY) for assistance with X-ray data collection. We thank R. Woodgate (NICDHD, NIH) for providing an expression clone for the Dbh protein. Crystallographic coordinates of a catalytic fragment of Dbh were kindly provided by T. Steitz, J. Pata and B.-L. Zhou (Yale University), and coordinates of S. cerevisiase Pol η were kindly provided by J. Tincao and A. Aggarwal (Mount Sinai School of Medicine).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silvian, L., Toth, E., Pham, P. et al. Crystal structure of a DinB family error-prone DNA polymerase from Sulfolobus solfataricus. Nat Struct Mol Biol 8, 984–989 (2001). https://doi.org/10.1038/nsb1101-984
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1101-984
This article is cited by
-
Structure and function relationships in mammalian DNA polymerases
Cellular and Molecular Life Sciences (2020)
-
DNA binding strength increases the processivity and activity of a Y-Family DNA polymerase
Scientific Reports (2017)
-
1H, 13C, and 15N backbone resonance assignments of the full-length 40 kDa S. acidocaldarius Y-family DNA polymerase, dinB homolog
Biomolecular NMR Assignments (2015)
-
Archaeal DNA polymerases in biotechnology
Applied Microbiology and Biotechnology (2015)
-
A nucleotide binding rectification Brownian ratchet model for translocation of Y-family DNA polymerases
Theoretical Biology and Medical Modelling (2011)