Abstract
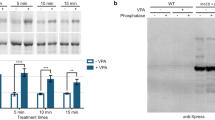
Serotonin N-acetyltransferase (arylalkylamine N-acetyltransferase, AANAT) controls daily changes in the production and circulating levels of melatonin. Here, the significance of the phosphorylation of AANAT was studied using a semisynthetic enzyme in which a nonhydrolyzable phosphoserine/threonine mimetic, phosphonomethylenealanine (Pma), was incorporated at position 31 (AANAT-Pma31). The results of studies in which AANAT-Pma31 and related analogs were injected into cells provide the first direct evidence that Thr31 phosphorylation controls AANAT stability in the context of the intact cells by binding to 14-3-3 protein. These findings establish Thr31 phosphorylation as an essential element in the intracellular regulation of melatonin production. The application of Pma in protein semisynthesis is likely to be broadly useful in the analysis of protein serine/threonine phosphorylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hunter, T. Signaling-2000 and beyond. Cell 100, 113–127 (2000).
Fu, H., Subramanian, R.R. & Masters, S.C. 14-3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40, 617–647 (2000).
Modrof, J. et al. Phosphorylation of Marburg virus VP30 at serines 40 and 42 is critical for its interaction with NP inclusions. Virology 287, 171–182 (2001).
Hao, M. et al. Mutation of phosphoserine 389 affects p53 function in vivo. J. Biol. Chem. 271, 29380–29385 (1996).
Lu, W., Shen, K. & Cole, P.A. Chemical dissection of the effects of tyrosine phosphorylation of SHP-2. Biochemistry 42, 5461–5468 (2003).
Zhang, Z., Shen, K., Lu, W. & Cole, P.A. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J. Biol. Chem. 278, 4668–4674 (2003).
Lu, W., Gong, D., Bar-Sagi, D. & Cole, P.A. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol. Cell 8, 759–769 (2001).
Klein, D.C. et al. 14-3-3 proteins in pineal photoneuroendocrine transduction: how many roles? J. Neuroendocrinol. 15, 370–377 (2003).
Ganguly, S. et al. Role of a pineal cAMP-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc. Natl. Acad. Sci. USA 98, 8083–8088 (2001).
Obsil, T., Ghirlando, R., Klein, D.C., Ganguly, S. & Dyda, F. Crystal structure of the 14-3-3ζ:serotonin N-acetyltransferase complex. a role for scaffolding in enzyme regulation. Cell 105, 257–267 (2001).
Dawson, P.E., Muir, T.W., Clark-Lewis, I. & Kent, S.B. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Erlanson, D.A., Chytil, M. & Verdine, G.L. The leucine zipper domain controls the orientation of AP-1 in the NFAT.AP-1.DNA complex. Chem. Biol. 3, 981–991 (1996).
Futaki, S. et al. Preparation of peptide thioesters using Fmoc-solid-phase peptide synthesis and its application to the construction of a template-assembled synthetic protein (TASP). Tetrahedron Lett. 38, 6237–6240 (1997).
Hackeng, T.M., Griffin, J.H. & Dawson, P.E. Protein synthesis by native chemical ligation: expanded scope by using straightforward methodology. Proc. Natl. Acad. Sci. USA 96, 10068–10073 (1999).
Hickman, A.B., Klein, D.C. & Dyda, F. Melatonin biosynthesis: the structure of serotonin N-acetyltransferase at 2.5 Å resolution suggests a catalytic mechanism. Mol. Cell 3, 23–32 (1999).
Ferry, G. et al. Characterization and regulation of a CHO cell line stably expressing human serotonin N-acetyltransferase (EC 2.3.1.87). Cell. Mol. Life Sci. 59, 1395–1405 (2002).
Gastel, J.A., Roseboom, P.H., Rinaldi, P.A., Weller, J.L. & Klein, D.C. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science 279, 1358–1360 (1998).
De Angelis, J., Gastel, J.A., Klein, D.C. & Cole, P.A. Kinetic analysis of the catalytic mechanism of serotonin N-acetyltransferase (EC 2.3.1.87). J. Biol. Chem. 273, 3045–3050 (1998).
Khalil, E.M., De Angelis, J., Ishii, M. & Cole, P.A. Mechanism-based inhibition of the melatonin rhythm enzyme: pharmacologic exploitation of active site functional plasticity. Proc. Natl. Acad. Sci. USA 96, 12418–12423 (1999).
Acknowledgements
We thank D.B. Murphy and C.J. Janetopoulos for advice on microinjection and immunofluorescence experiments. We are grateful for financial support from the US National Institutes of Health and the Ellison Medical Foundation. Mass spectrometry analysis was carried out in the AB Mass Spectrometry/Proteomics Facility at Johns Hopkins School of Medicine with support from a US National Center for Research Resources shared instrumentation grant, the Johns Hopkins Fund for Medical Discovery and the Institute for Cell Engineering.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zheng, W., Zhang, Z., Ganguly, S. et al. Cellular stabilization of the melatonin rhythm enzyme induced by nonhydrolyzable phosphonate incorporation. Nat Struct Mol Biol 10, 1054–1057 (2003). https://doi.org/10.1038/nsb1005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb1005
This article is cited by
-
Synthesis of Phosphopeptides in the Fmoc Mode
International Journal of Peptide Research and Therapeutics (2007)