Abstract
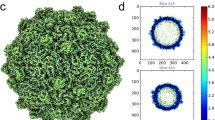
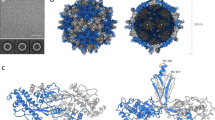
The three-dimensional structure of rice dwarf virus was determined to 6.8 Å resolution by single particle electron cryomicroscopy. By integrating the structural analysis with bioinformatics, the folds of the proteins in the double-shelled capsid were derived. In the outer shell protein, the uniquely orientated upper and lower domains are composed of similar secondary structure elements but have different relative orientations from that of bluetongue virus in the same Reoviridae family. Differences in both sequence and structure between these proteins may be important in defining virus–host interactions. The inner shell protein adopts a conformation similar to other members of Reoviridae, suggesting a common ancestor that has evolved to infect hosts ranging from plants to animals. Symmetry mismatch between the two shells results in nonequivalent, yet specific, interactions that contribute to the stability of this large macromolecular machine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gouet, P. et al. Cell 97, 481–490 (1999).
Lawton, J.A., Estes, M.K. & Prasad, B.V. Nature Struct. Biol. 4, 118–121 (1997).
Nibert, M.L., Schiff, L.A. & Field, B.N. in Fields Virology, Vol. 2 (eds. Fields, B.N. et al.) 1557–1596 (Lippincott-Raven, Philadelphia; 1996).
Lu, G. et al. J. Virol. 72, 8541–8549 (1998).
Zhu, Y. et al. J. Virol. 71, 8899–8901. (1997).
Wu, B. et al. Virology 271, 18–25. (2000).
Shao, C., Zhou, Z.H. & Lu, G. Science in China 44, 192–198 (2001).
Zhou, Z.H. et al. Science 288, 877–880 (2000).
Jiang, W., Baker, M.L., Ludtke, S.J. & Chiu, W. J. Mol. Biol. 308, 1033–1044 (2001).
Grimes, J.M. et al. Nature 395, 470–477 (1998).
Reinisch, K.M., Nibert, M.L. & Harrison, S.C. Nature 404, 960–967. (2000).
McGuffin, L.J., Bryson, K. & Jones, D.T. Bioinformatics 16, 404–405. (2000).
Rost, B., Sander, C. & Schneider, R. Comput. Appl. Biosci. 10, 53–60. (1994).
Baldi, P., Brunak, S., Frasconi, P., Soda, G. & Pollastri, G. Bioinformatics 15, 937–946. (1999).
Mizuguchi, K. & Go, N. Protein Eng. 8, 353–362. (1995).
Kleywegt, G.J. & Jones, T.A. Methods Enzymol. 277, 525–545 (1997).
Fisher, D. & Eisenberg, D. Protein Sci. 5, 947–955 (1996).
Grimes, J.M., Basak, A.K., Roy, P. & Stuart, D.I. Nature 373, 167–170 (1995).
Grimes, J.M. et al. Structure 5, 885–893 (1997).
Pesavento, J.B., Lawton, J.A., Estes, M.K. & Prasad, B.V. Proc. Natl. Acad. Sci. USA 98, 1381–1386. (2001).
Zhang, H. et al. J. Virol. 73, 1624–1629. (1999).
Fuller, S.D. Cell 48, 923–934 (1987).
Zhou, Z.H. et al. Nature Struct. Biol. 2, 1026–1030 (1995).
Zhou, Z.H. et al. Biophys. J. 74, 576–588 (1998).
Zhou, Z.H., Chen, D.H., Jakana, J., Rixon, F.J. & Chiu, W. J. Virol. 73, 3210–3218 (1999).
Crowther, R.A. Phil. Trans. R Soc. Lond. B Biol. Sci. 261, 221–230 (1971).
van Heel, M. Ultramicroscopy 21, 95–100 (1987).
Böttcher, B., Wynne, S.A. & Crowther, R.A. Nature 386, 88–91 (1997).
He, J., Schmid, M.F., Zhou, Z.H., Rixon, F. & Chiu, W. J. Mol. Biol. 309, 903–914 (2001).
Ludtke, S.J., Baldwin, P.R. & Chiu, W. J. Struct. Biol. 128, 82–97 (1999).
Acknowledgements
This research has been supported by grants from National Institutes of Health, the Robert Welch Foundation and the National Natural Science Foundation of China. Z.H.Z. is a Pew Scholar in the Biomedical Sciences and a Basil O'Connor Starter Scholar of the March of Dimes Foundation. M.L.B. was supported in part by Baylor Research Advocates for Student. We would like to thank B.V.V. Prasad, M.F. Schmid and M. Baker for their helpful comments on the manuscript. Supplementary animations and 3D VRML models are available at http://ncmi.bcm.tmc.edu/~wjiang/rdv/.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Baker, M., Jiang, W. et al. Electron cryomicroscopy and bioinformatics suggest protein fold models for rice dwarf virus. Nat Struct Mol Biol 8, 868–873 (2001). https://doi.org/10.1038/nsb1001-868
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1001-868
This article is cited by
-
Cryo-EM reconstruction of the Cafeteria roenbergensis virus capsid suggests novel assembly pathway for giant viruses
Scientific Reports (2017)
-
Double-stranded RNA virus outer shell assembly by bona fide domain-swapping
Nature Communications (2017)
-
Transcriptome profiling confirmed correlations between symptoms and transcriptional changes in RDV infected rice and revealed nucleolus as a possible target of RDV manipulation
Virology Journal (2014)
-
Reduction of the secondary structure topological space through direct estimation of the contact energy formed by the secondary structures
BMC Bioinformatics (2009)
-
3D electron microscopy of biological nanomachines: principles and applications
European Biophysics Journal (2007)