Abstract
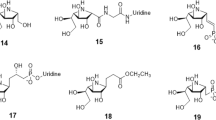
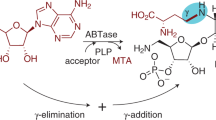
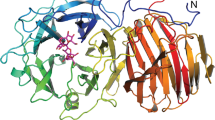
Uridine diphosphogalactofuranose (UDP-Galf ) is the precursor of the d-galactofuranose (Galf ) residues found in bacterial and parasitic cell walls, including those of many pathogens, such as Mycobacterium tuberculosis and Trypanosoma cruzi. UDP-Galf is made from UDP-galactopyranose (UDP-Galp) by the enzyme UDP-galactopyranose mutase (mutase). The mutase enzyme is essential for the viability of mycobacteria and is not found in humans, making it a viable therapeutic target. The mechanism by which mutase achieves the unprecedented ring contraction of a nonreducing sugar is unclear. We have solved the crystal structure of Escherichia coli mutase to 2.4 Å resolution. The novel structure shows that the flavin nucleotide is located in a cleft lined with conserved residues. Site-directed mutagenesis studies indicate that this cleft contains the active site, with the sugar ring of the substrate UDP-galactose adjacent to the exposed isoalloxazine ring of FAD. Assay results establish that the enzyme is active only when flavin is reduced. We conclude that mutase most likely functions by transient reduction of substrate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Besra, G.S. et al. Biochemistry 34, 4257–4266 (1995).
Pan, F. Jackson, M., Ma, Y. & McNeil, M. J. Bacteriol. 183 3991–3998 (2001).
Knirel, Y.A. & Kochetkov, N.L. Biochemistry (Moscow) 59, 1325–1383 (1994).
Joiner, K.A. Annu Rev. Microbiol. 42, 201–230 (1988).
Turco, S.J. et al. J. Biol. Chem. 264, 6711–6715 (1989).
McConville, M.J., Thomas-Oates, J.E., Ferguson, M.A.J. & Homans, S.W. J. Biol. Chem. 265, 19611–19623 (1990).
Ilg, T. et al. J. Biol. Chem. 267, 6834–6840 (1992).
Spath, G.F. et al. Proc. Natl. Acad. Sci. USA 97, 9258–9263 (2000).
Previato, J.O. et al. J. Biol. Chem. 265, 2518–2526 (1990).
Parra, E. et al. Carbonhydr. Res. 257, 239–248 (1994).
Takayanagi, T., Kimura, A., Chiba, S. & Ajisaka, K. Carbohydr. Res. 256, 149–158 (1994).
Nakajima, T., Yoshida, M., Nakamura, M., Hiura, N. & Matsuda, K. J. Biochem. 96, 1013–1020 (1984).
Nassau, P.M. et al. J. Bacteriol. 178, 1047–1052 (1996).
Koplin, R., Brisson, J.-R. & Whitfield, C. J. Biol. Chem. 272, 4121–4128 (1997).
Barlow, J.N., Marcinkeviciene, J. & Blanchard, J.S. in Enzymatic mechanisms (eds Frey, P.A. & Northrop, D.B.) 98–106 (IOS Press, Amsterdam; 1999).
Zhang, Q. & Liu, H.-W. J. Am. Chem. Soc. 122, 9065–9070 (2000).
Barlow, J.N., Girvin, M.E. & Blanchard, J.S. J. Am. Chem. Soc. 121, 6968–6969 (1999).
Barlow, J.N. & Blanchard, J.S. Carbohydr. Res. 328, 473–480 (2000).
McMahon, S.A., Leonard, G.L., Buchanan, L.V., Giraud, M.-F. & Naismith, J.H. Acta Crystallogr. D 55, 399–402 (1999).
Jones, S. & Thornton, J.M. Proc. Natl. Acad. Sci. USA 93, 13–20 (1996).
Holm, L. & Sanders, C. J. Mol. Biol. 233, 123–138 (1993).
Fraaije, M.W. & Mattevi, A. TIBS 25, 126–132 (2000).
Karplus, P.A. & Schulz, G.E. J. Mol. Biol. 210, 163–180 (1989).
Hunter, W.N. et al. J. Mol. Biol. 227, 322–333 (1992).
Artymiuk, P.J., Poirrette, A.R., Grindley, H.M., Rice, D.W. & Willett, P. J. Mol. Biol. 243, 327–344 (1994).
Charnock, S.J. & Davies, G.J. Biochemistry 38, 6380–6385 (1999).
Gastinel, L.N., Cambillau, C. & Bourne, Y. EMBO J. 18, 3546–3557 (1999).
Busch, C., Hofmann, F., Gerhard, R. & Aktories, K. J. Biol. Chem. 275, 13228–13234 (2000).
Hill, S. Austin, S. Eydmann, T., Jones, T. & Dixon, R. Proc. Natl. Acad. Sci. USA 93, 2143–2148 (1996)
Sanders, D.A.R., McMahon, S.A., Leonard, G.L. & Naismith, J.H. Acta Crystallogr. D In the press (2001).
Brünger, A.T., et al. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Lee, R. et al. Anal. Biochem. 242, 1–7 (1996).
Esnouf, R.M. J. Mol. Graph. Model. 15,132–134 (1997).
Gouet, P., Courcelle, E., Stuart, D.I. & Metoz, F. Bioinformatics 15, 305–308 (1999).
Nicholls, A., Sharp, K. & Honig, B. Proteins 11, 281–296 (1991).
Acknowledgements
The work is supported by the BBSRC. We thank W. Crocker, S. Chapman, S. Daff, R. Harris, N. Scrutton and G. Leonard for their help with experiments. We thank the reviewers and editor for assistance in revising the manuscript. We thank J. Blanchard and J. Barlow for helpful discussions. J.H.N. is a BBSRC Career Development Fellow. C.W. is a CIHR Senior Scientist and is supported by the Canadian Bacterial Diseases Network (NCE program).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanders, D., Staines, A., McMahon, S. et al. UDP-galactopyranose mutase has a novel structure and mechanism. Nat Struct Mol Biol 8, 858–863 (2001). https://doi.org/10.1038/nsb1001-858
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb1001-858
This article is cited by
-
Complete genome determination and analysis of Acholeplasma oculi strain 19L, highlighting the loss of basic genetic features in the Acholeplasmataceae
BMC Genomics (2014)
-
Protein Separation and Enzyme Purification by Preparative Capillary Isotachophoresis
Chromatographia (2013)
-
Alteration of the exopolysaccharide production and the transcriptional profile of free-living Frankia strain CcI3 under nitrogen-fixing conditions
Applied Microbiology and Biotechnology (2013)
-
Defining mycobacteria: Shared and specific genome features for different lifestyles
Indian Journal of Microbiology (2009)
-
A unique catalytic mechanism for UDP-galactopyranose mutase
Nature Structural & Molecular Biology (2004)