Abstract
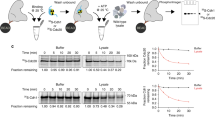
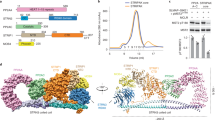
The anaphase-promoting complex (APC), or cyclosome, is a cell cycle-regulated ubiquitin ligase that controls progression through mitosis and the G1 phase of the cell cycle. The APC is composed of at least 11 subunits; no structure has been determined for any of these subunits. The subunit APC10/DOC1, a one-domain protein consisting of 185 amino acids, has a conserved core (residues 22–161) that is homologous to domains found in several other putative ubiquitin ligases and, therefore, may play a role in ubiquitination reactions. Here we report the crystal structure of human APC10 at 1.6 Å resolution. The core of the protein is formed by a β-sandwich that adopts a jellyroll fold. Unexpectedly, this structure is highly similar to ligand-binding domains of several bacterial and eukaryotic proteins, such as galactose oxidase and coagulation factor Va, raising the possibility that APC10 may function by binding a yet unidentified ligand. We further provide biochemical evidence that the C-terminus of APC10 binds to CDC27/APC3, an APC subunit that contains multiple tetratrico peptide repeats.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Peters, J.M. Exp. Cell Res. 248, 339–349 (1999).
Gmachl, M., Gieffers, C., Podtelejnikov, A.V., Mann, M. & Peters, J.M. Proc. Natl. Acad. Sci. USA 97, 8973–8978 (2000).
Leverson, J.D. et al. Mol. Biol. Cell 11, 2315–2325 (2000).
Hwang, L.H. & Murray, A.W. Mol. Biol. Cell 8, 1877–1887 (1997).
Zachariae, W. et al. Science 279, 1216–1219 (1998).
Kominami, K., Seth-Smith, H. & Toda, T. EMBO J. 17, 5388–5399 (1998).
Grossberger, R. et al. J. Biol. Chem. 274, 14500–14507 (1999).
Pravtcheva, D.D. & Wise, T.L. Genomics 72, 78–87 (2001).
Gieffers, C., Schleiffer, A. & Peters, J.-M. Protoplasma 211, 20–28 (2000).
Weissmann, A.M. Nat. Rev. Mol. Cell Biol. 2, 169–178 (2001).
Ito, N., Phillips, S.E., Yadav, K.D. & Knowles, P.F. J. Mol. Biol. 238, 794–814 (1994).
Gaskell, A., Crennell, S. & Taylor, G. Structure 3, 1197–1205 (1995).
Macedo-Ribeiro, S. et al. Nature 402, 434–439 (1999).
Marintchev, A. et al. Nature Struct. Biol. 6, 884–893 (1999).
Baumgartner, S., Hofmann, K., Chiquet-Ehrismann, R. & Bucher, P. Protein Sci. 7, 1626–1631 (1998).
Scheufler, C. et al. Cell 101, 199–210 (2000).
Gatto, G.J., Geisbrecht, B.V., Gould, S.J. & Berg, J.M. Nature Struct. Biol. 7, 1091–1095 (2000).
Gieffers, C., Dube, P., Harris, J.R., Stark, H. & Peters, J.-M. Mol. Cell. 7, 907–913 (2001).
Brinkmann, U., Mattes, R.E. & Buckel, P. Gene 85, 109–114 (1989).
Budisa, N. et al. Eur. J. Biochem. 230, 788–796 (1995).
Otwinowski Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Collaborative Computational Project, Number 4. Acta Crystallogr. D 50, 760–763 (1994).
Cowtan, K. In Joint CCP4 and ESD-EACBM Newsletter on Protein Crystallography 31, 34–38 (1994).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard . Acta Crystallogr. A 47, 110–119 (1991).
Brunger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Laskowski, R.A., Rullmann, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M. J. Biomol. NMR 8, 477–486 (1996).
Holm, L. & Sander, C. J. Mol. Biol. 233, 123–138 (1993).
Lachner, M., O'Carroll, D., Rea, S., Mechtler, K. & Jenuwein, T. Nature 410, 116–120 (2001).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Merrit, E.A. & Murphy, M.E.P. Acta Crystallogr. D 50, 869–873 (1994).
Esnouf, R. J. Mol. Graphics 15, 132–134 (1997).
Nicholls, A., Bharadwaj, R. & Honig, B. Biophys. J. 64, A166 (1993).
Barton, G.J. Protein Eng. 6, 37–40 (1993).
Acknowledgements
The assistance of G.P. Bourenkov and H. Bartunik at DESY, Hamburg for data recording is greatly acknowledged. We thank K. Mechtler for peptide synthesis and M. Lachner for the histone H4 N-terminal peptide matrix. Research in the laboratory of J.-M. P. is supported by Boehringer Ingelheim and by grants from the Austrian Science Fund and the Austrian Industrial Research Promotion Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wendt, K., Vodermaier, H., Jacob, U. et al. Crystal structure of the APC10/DOC1 subunit of the human anaphase-promoting complex. Nat Struct Mol Biol 8, 784–788 (2001). https://doi.org/10.1038/nsb0901-784
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0901-784
This article is cited by
-
The ZZ domain of HERC2 is a receptor of arginylated substrates
Scientific Reports (2022)
-
The RIO protein kinase-encoding gene Sj-riok-2 is involved in key reproductive processes in Schistosoma japonicum
Parasites & Vectors (2017)
-
Atomic structure of the APC/C and its mechanism of protein ubiquitination
Nature (2015)
-
Molecular architecture and mechanism of the anaphase-promoting complex
Nature (2014)
-
Mutually dependent degradation of Ama1p and Cdc20p terminates APC/C ubiquitin ligase activity at the completion of meiotic development in yeast
Cell Division (2013)