Abstract
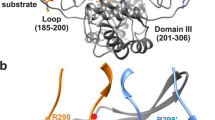
HIV-1 proteinase (HIV PR) is a dimeric enzyme composed of two identical polypeptide chains that associate with twofold symmetry. We have determined to 1.8 Å the crystal structure of a covalently tethered dimer of HIV PR. The tethered dimer:inhibitor complex is identical in nearly every respect to the complex of the same inhibitor with the wild type dimeric molecule, except for the linker region. Our results suggest that the tethered dimer may be a useful surrogate enzyme for studying the effects of single site mutations on substrate and inhibitor binding as well as on enzyme asymmetry, and for simulating independent mutational drift of the two domains which has been proposed to have led to the evolution of modern day, single-chain aspartic proteinases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Huff, J.R. HIV protease: a novel chemotherapeutic target for AIDS J. med. Chem. 34, 2305–2314 (1991)
Meek, T.D. Inhibitors of HIV-1 protease J. Enzym. Inhib. 6, 65–98 (1992)
Norbeck, D.W. & Kempf, D.J. HIV protease inhibitors. Ann. Rep. med. Chem. 26, 141–150 (1991)
Tomasselli, A.G., Howe, W.J., Sawyer, T.K., Wlodawer, A. & Heinrikson, R.L. The complexities of AIDS: an assessment of the HIV protease as a therapeutic target. Chimica Oggi 6–27 (1991)
Erickson, J.W. Design and structure of symmetry-based inhibitors of HIV-1 protease. Perspect. drug dis. Design 1, 109–128(1993)
Otto, M.J. et al. In vitro isolation and identification of human immunodeficiency virus (HIV) variants with reduced sensitivity to C-2 symmetrical inhibitors of HIV type 1 protease. Proc. natn. Acad. Sci. U.S.A. 90, 7543–7547 (1993)
Ho, D.D. et al. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J. Virol. 68, 2016–2020 (1994)
Kaplan, A.H. et al. Selection of HIV-1 variants which encode viral proteases with decreased sensitivity to an inhibitor of the viral protease. Proc. natn. Acad. Sci. USA. (in the press 1994).
Cheng, Y.-S.E., Yin, F.H., Foundling, S., Blomstrom, D. & Kettner, C.A. Stability and activity of human immunodeficiency virus protease: comparison of the natural dimer with a homologous, single-chain tethered dimer. Proc. natn. Acad. Sci. U.S.A. 87, 9660–9664 (1990)
Dilanni, C.L. et al. Characterization of an active single polypeptide form of the human immunodeficiency virus type 1 protease. J. biol. Chem. 265, 17348–17354 (1990)
Kräusslich, H.-G. Human immunodeficiency virus proteinase dimer as component of the viral polyprotein prevents particle assembly and viral infectivity. Proc. natn. Acad. Sci. U.S.A. 88, 3213–3217 (1991)
Griffiths, J. et al. Interactions of substrates and inhibitors with a family of tethered HIV-1 and HIV-2 Homo- and Heterodimers. J. biol. Chem. 269, 4787–4793 (1994)
Hosur, M.V. et al. Influence of stereochemistry on activity and binding modes for C2 symmetric-based diol inhibitors of HIV-1 protease. J. Am. chem. Soc. 116, 848–855 (1994)
Wlodawer, A. & Erickson, J.W. Structure-based inhibitors of HIV-1 protease.. Rev. Biochem. 62, 543–585 (1993)
Topol, I.A., Cachau, R.E., Burt, S.K. & Erickson, J.W. in Aspartic Proteinases. (ed. Takahashi, K.) (Plenum Press, New York, 1994).
Rao, J.K.M., Erickson, J.W. & Wlodawer, A. Structural and evolutionary relationships between retroviral and eucaryotic aspartic proteinases. Biochemistry 30, 4663–4671 (1991)
Tang, J. & Wong, R. Evolution in the structure and function of aspartic proteases. J. cell. Biochem. 33, 53–63 (1987)
Kuzmic, P. Kinetic assay for HIV proteinase subunit dissociation. Biochem. biophys. Res. Comm. 191, 998–1003 (1993)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhat, T., Baldwin, E., Liu, B. et al. Crystal structure of a tethered dimer of HIV-1 proteinase complexed with an inhibitor. Nat Struct Mol Biol 1, 552–556 (1994). https://doi.org/10.1038/nsb0894-552
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0894-552
This article is cited by
-
A single mutation in the core domain of the lac repressor reduces leakiness
Microbial Cell Factories (2013)