Abstract
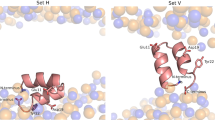
The N-terminal domain of the influenza hemagglutinin (HA) is the only portion of the molecule that inserts deeply into membranes of infected cells to mediate the viral and the host cell membrane fusion. This domain constitutes an autonomous folding unit in the membrane, causes hemolysis of red blood cells and catalyzes lipid exchange between juxtaposed membranes in a pH-dependent manner. Combining NMR structures determined at pHs 7.4 and 5 with EPR distance constraints, we have deduced the structures of the N-terminal domain of HA in the lipid bilayer. At both pHs, the domain is a kinked, predominantly helical amphipathic structure. At the fusogenic pH 5, however, the domain has a sharper bend, an additional 310-helix and a twist, resulting in the repositioning of Glu 15 and Asp 19 relative to that at the nonfusogenic pH 7.4. Rotation of these charged residues out of the membrane plane creates a hydrophobic pocket that allows a deeper insertion of the fusion domain into the core of the lipid bilayer. Such an insertion mode could perturb lipid packing and facilitate lipid mixing between juxtaposed membranes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Steinhauer, D.A., Wharton, S.A., Skehel, J.J. & Wiley, D.C. Studies of membrane fusion activities of fusion peptide mutants of influenza virus hemagglutinin. J. Virol. 69, 6643–6651 (1995).
Delahunty, M.D., Rhee, I., Freed, E.O. & Bonifacino, J.S. Mutational analysis of the fusion peptide of the human immunodeficiency virus type 1: identification of critical glycine residues. Virology 218, 94–102 (1996).
Qiao, H., Armstrong, R.T., Melikyan, G.B., Cohen, F.S. & White, J.M. A specific point mutant at position 1 of the influenza hemagglutinin fusion peptide displays a hemifusion phenotype. Mol. Biol. Cell 10, 2759–2769 (1999).
Wilson, I.A., Skehel, J.J. & Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289, 366–372 (1981).
Bullough, P.A., Hughson, F.M., Skehel, J.J. & Wiley, D.C. Structure of influenza haemagglutinin at the pH of membane fusion. Nature 317, 37–43 (1994).
Fass, D., Harrison, S.C. & Kim, P.S. Retrovirus envelope domain at 1.7 Å resolution. Nature Struct. Biol. 3, 465–469 (1996).
Weissenhorn, W., Dessen, A., Harrison, S.C., Skehel, J.J. & Wiley, D.C. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387, 426–430 (1997).
Weissenhorn, W., Carfí, A., Lee, K.H., Skehel, J.J. & Wiley, D.C. Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain. Mol. Cell 2, 605–616 (1998).
Malashkevich, V.N., Chan, D.C., Chutkowski, C.T. & Kim, P.S. Crystal structure of the simian immunodeficiency virus (SIV) gp41 core: conserved helical interactions underlie the broad inhibitory activity of gp41 peptides. Proc. Natl. Acad. Sci. USA 95, 9134–9139 (1998).
Caffrey, M., et al. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 17, 4572–4584 (1998).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brünger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 (1998).
Han, X. & Tamm, L.K. A host-guest system to study structure-function relationships of membrane fusion peptides. Proc. Natl. Acad. Sci. USA 97, 13097–13102 (2000).
Han, X. & Tamm, L.K. pH-dependent self-association of influenza hemagglutinin fusion peptides in lipid bilayers. J. Mol. Biol. 304, 953–965 (2000).
Güntert, P., Braun, W. & Wüthrich, K. Efficient computation of three-dimensional protein structures in solution from nuclear magnetic resonance data using the program DIANA and the supporting programs CALIBA, HABAS and GLOMSA. J. Mol. Biol. 217, 517–530 (1991).
Altenbach, C., Greenhalgh, D.A., Khorana, H.G. & Hubbell, W.L. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc. Natl. Acad. Sci. USA 91, 1667–1671 (1994).
Tamm, L.K. & Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 30, 365–429 (1997).
Macosko, J.C., Kim C.-H. & Shin, Y.-K. The membrane topology of the fusion peptide region of influenza hemagglutinin determined by spin-labeling EPR. J. Mol. Biol. 267, 1139–1148 (1997).
Tamm, L.K. & Han, X. Viral fusion peptides: a tool set to disrupt and connect biological membranes. Biosience Reports 20, 501–518 (2000).
Armstrong, R.T., Kushnir, A.S. & White, J.M. The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J. Cell Biol. 151, 425–438 (2000).
Tatulian, S.A. & Tamm, L.K. Secondary structure, orientation, oligomerization, and lipid interactions of the transmembrane domain of influenza hemagglutinin. Biochemistry 39, 496–507 (2000).
Güntert, P., Mumenthaler, C. & Wüthrich, K. Torsion angle dynamics for NMR structure calculation with the new program DYANA. J. Mol. Biol. 273, 283–298 (1997).
Luginbühl, P., Güntert, P., Billeter, M. & Wüthrich, K. The new program OPAL for molecular dynamics simulations and energy refinements of biological macromolecules. J. Biomol. NMR 8, 136–146 (1996).
Frey, S. & Tamm, L.K. Orientation of melittin in phospholipid bilayers. A polarized attenuated total reflection infrared study. Biophys. J. 60, 922–930 (1991).
Tamm, L.K. & Tatulian, S.A. Orientation of functional and nonfunctional PTS permease signal sequences in lipid bilayers. A polarized attenuated total reflection infrared study. Biochemistry 32, 7720–7726 (1993).
Dalton, L.A., McIntyre, J.O. & Fleischer, S. Distance estimate of the active center of D-β-hydroxybutyrate dehydrogenase from the membrane surface. Biochemistry 26, 2117–2130 (1987).
Langen, R., Oh, K.J., Cascio, D. & Hubbell, W.L. Crystal structures of spin labeled T4 lysozyme mutants: implications for the interpretation of EPR spectra in terms of structure. Biochemistry 39, 8396–8405 (2000).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
White, S.H. & Wiener, M.C. In Membrane structure and dynamics (eds. Merz, K.M. & Roux, B.) 127–144 (Birkhäuser, Boston; 1996).
Laskowski, R.A., Rullmann, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486 (1996).
Acknowledgements
We thank K. Victor for his expert advice on the EPR measurements and fitting procedures. This work was supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X., Bushweller, J., Cafiso, D. et al. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat Struct Mol Biol 8, 715–720 (2001). https://doi.org/10.1038/90434
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/90434
This article is cited by
-
Peptide-mediated delivery of CRISPR enzymes for the efficient editing of primary human lymphocytes
Nature Biomedical Engineering (2023)
-
Planar aggregation of the influenza viral fusion peptide alters membrane structure and hydration, promoting poration
Nature Communications (2022)
-
Mechanism of Membrane Fusion: Interplay of Lipid and Peptide
The Journal of Membrane Biology (2022)
-
Why does the Aβ peptide of Alzheimer share structural similarity with antimicrobial peptides?
Communications Biology (2020)
-
Membrane Composition Modulates Fusion by Altering Membrane Properties and Fusion Peptide Structure
The Journal of Membrane Biology (2019)