Abstract
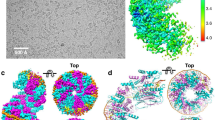
The structure of the homotetrameric DNA binding domain of the single stranded DNA binding protein from Escherichia coli (Eco SSB) bound to two 35-mer single stranded DNAs was determined to a resolution of 2.8 Å. This structure describes the vast network of interactions that results in the extensive wrapping of single stranded DNA around the SSB tetramer and suggests a structural basis for its various binding modes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Meyer, R.R. & Laine, P.S. Microbiol. Rev. 54, 342–380 (1990).
Lohman, T.M. & Ferrari, M.E. Annu. Rev. Biochem. 63, 527–570 (1994).
Wold, M.S. Annu. Rev. Biochem. 66, 61–92 (1997).
Chase, J.W. & Williams, K.R. Annu. Rev. Biochem. 55, 103–136 (1986).
Lohman, T.M. & Overman, L.B. J. Biol. Chem. 260, 3594–3603 (1985).
Bujalowski, W. & Lohman, T.M. Biochemistry 25, 7799–7802 (1986).8.
Chrysogelos, S. & Griffith, J. Proc. Natl. Acad. Sci. USA 79, 5803–5807 (1982).
Lohman, T.M., Overman, L.B. & Datta, S. J. Mol. Biol. 187, 603–615 (1986).
Griffith, J.D., Harris, L.D. & Register, J. Cold Spring Harbor Symp. Quant. Biol. 49, 553–559 (1984).
Lohman, T.M. & Bujalowski, W. Biochemistry 27, 2260–2265 (1988).
Bujalowski, W. & Lohman, T.M. J. Mol. Biol. 207, 269–288 (1989).
Raghunathan, S., Ricard, C.S., Lohman, T.M. & Waksman, G. Proc. Natl. Acad. Sci. USA 94, 6652–6657 (1997).
Curth, U., Greipel, J., Urbanke, C. & Maass, G. Biochemistry 32, 2585–2591 (1993).
Khamis, M.I., Casas-Finet, J.R., Maki, A.H., Murphy, J.B. & Chase, J.W. J. Biol. Chem. 262, 10938–10945 (1987).
Ferrari, M.E., Fang, J. & Lohman, T.M. Biophys. Chem. 64, 235–251 (1997).
Merrill, B.M., Williams, K.R., Chase, J.W. & Konigsberg, W.H. J. Biol. Chem. 259, 10850–10856 (1984).
Casas-Finet, J.R., Khamis, M.I., Maki, A.H. & Chase, J.W. FEBS Lett. 220, 347–352 (1987).
Bayer, I., Fliess, A., Greipel, J., Urbanke, C. & Maass, G. Eur. J. Biochem. 179, 399–404 (1989).
Schmellik-Sandage, C.S. & Tessman, E.S. J. Bacteriol. 172, 4378–4385 (1990).
Overman, L.B., Bujalowski, W. & Lohman, T.M. Biochemistry 27, 456–471 (1988).
Chen, J., Smith, D.L. & Griep, M.A. Protein Sci. 7, 1781–1788 (1998).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Terwilliger, T.C., Kim, S.-H. & Eisenberg, D. Acta Crystallogr. A 43, 1–5 (1987).
De La Fortelle, E. & Bricogne, G. Methods Enzymol. 276, 472–493 (1997).
Abrahams, J.P. & Leslie, A.G.W. Acta Crystallogr. D 52, 32–42 (1996).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Brünger, A.T. Nature 355, 472–474 (1992).
Acknowledgements
G.W. and T.M.L were supported in this work by the NIH. We gratefully acknowledge T. Ho for synthesis and purification of oligodeoxynucleotides, S. Korolev and K. Fütterer for help during data collection, and the staff of beamline BL9.1 of SSRL, beamline X4A of NSLS, and beamline 19ID of APS.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Raghunathan, S., Kozlov, A., Lohman, T. et al. Structure of the DNA binding domain of E. coli SSB bound to ssDNA. Nat Struct Mol Biol 7, 648–652 (2000). https://doi.org/10.1038/77943
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/77943
This article is cited by
-
Replication fork binding triggers structural changes in the PriA helicase that govern DNA replication restart in E. coli
Nature Communications (2023)
-
Towards a better understanding of antimicrobial resistance dissemination: what can be learnt from studying model conjugative plasmids?
Military Medical Research (2022)
-
Crystal structure of the INTS3/INTS6 complex reveals the functional importance of INTS3 dimerization in DSB repair
Cell Discovery (2021)
-
Resonance assignments and secondary structure of thermophile single‐stranded DNA binding protein from Sulfolobus solfataricus at 323K
Biomolecular NMR Assignments (2021)
-
MutL sliding clamps coordinate exonuclease-independent Escherichia coli mismatch repair
Nature Communications (2019)