Abstract
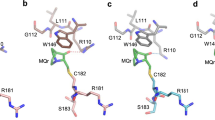
A Ca2+ dependent conformational change in dimeric S100B(ββ) is required for it to bind p53 and inhibit phosphorylation of this tumor suppressor in its C-terminal negative regulatory domain. A peptide derived from this region of p53 (residues 367–388) was found to have no regular structure in its native form by NMR spectroscopy, but becomes helical when bound to Ca2+ loaded S100B(ββ). The three-dimensional structure of this complex reveals several favorable hydrophobic and electrostatic interactions between S100B(ββ) and the p53 peptide in the binding pocket, where S100B(ββ) sterically blocks sites of phosphorylation and acetylation on p53 that are important for transcription activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Levine, A.J. Cell 88, 323–331 (1997).
Cho, Y., Gorina, S., Jeffrey, P.D. & Pavletich, N.P. Science 265, 346–355 (1994).
Jeffrey, P.D., Gorina, S. & Pavletich, N.P. Science 267, 1498– 1502 (1995).
Lee, W., et al. Nature Struct. Biol. 1, 877–890 (1994).
Clore, G.M., et al. Nature Struct. Biol. 2, 321–333 (1995).
Sakaguchi, K., et al. Genes Dev. 12, 2831–2841 (1998).
Baudier, J., Delphin, C., Grundwald, D., Khochbin, S. & Lawrence, J.J. Proc. Natl. Acad. Sci. USA 89, 11627–11631 (1992).
Scotto, C., Deloulme, J.C., Rousseau, D., Chambaz, E. & Baudier, J. Mol. Cell Biol. 18, 4272– 4281 (1998).
Wilder, P.T., Rustandi, R.R., Drohat, A.C. & Weber, D.J. Protein Sci. 7, 794–798 (1998).
Carrier, F., Blake, M., Zimmer, D., Rustandi, R.R. & Weber, D.J. Proc. Amer. Assoc. Cancer Res. 40, 102 (1999).
Kligman, D. & Hilt, D. Trends Biochem. Sci. 13 , 437–443 (1988).
Smith, S.P. & Shaw, G.S. Structure 6, 211–222 (1998).
Drohat, A.C., Baldisseri, D.M., Rustandi, R.R. & Weber, D.J. Biochemistry 37, 2729–2740 (1998).
Drohat, A.C., Tjandra, N., Baldisseri, D.M. & Weber, D.J. Protein Sci. 8, 800–809 (1999).
Matsumura, H., Shiba, T., Inoue, T., Harada, S. & Kay, Y. Structure 6, 233–241 ( 1998).
Rustandi, R.R., Drohat, A.C., Baldisseri, D.M., Wilder, P.T. & Weber, D.J. Biochemistry 37, 1951–1960 (1998).
Rustandi, R.R., Baldisseri, D.M., Drohat, A.C. & Weber, D.J. Protein Sci. 8, 1743–1751 (1999).
Drohat, A.C., Nenortas, E., Beckett, D. & Weber, D.J. Protein Sci. 6, 1577–1582 (1997).
Li, M.X., Spyracopoulos, L., Sykes, B.D. Biochemistry 38, 8289–8298 (1999).
Donato, R. Biochim. Biophys. Acta 1450, 191–231 ( 1999).
Rety, S., et al. Struct. Fold. Des. 15, 175–184 (2000).
Rety, S., et al. Nature Struct. Biol. 6, 89–95 (1999).
Wishart, D.S., Sykes, B.D. & Richards, F.M. Biochemistry 31, 1647– 1651 (1992).
Ivanenkov, V.V., Jamieson, G.A., Gruenstein, E. & Dimlich, R.V.W. J. Biol. Chem. 270, 14651–14658 (1995).
Youmell, M., Park, S.J., Basu, S. & Price, B.D. Biochem. Biophys. Res. Comm. 245, 514–518 (1998).
Davison, T. S., Yin, P., Nie, E., Kay, C. & Arrowsmith, C.H. Oncogene 17, 651– 656 (1998).
Sakaguchi, K. et al. Biochemistry 36, 10117–10124 (1997).
Delphin, C. et al. J. Biol. Chem. 274, 10539–10544 (1999).
Bax, A., Grzesiek, S., Gronenborn, A.M. & Clore, G.M. J. Magn. Reson. A 106, 269–273 ( 1994).
Vuister, G.W., Kim, S.-J., Wu, C. & Bax, A. J. Am. Chem. Soc. 116, 9206–9210 ( 1994).
Brünger, A.T. X-PLOR Version 3.1. (Yale University Press, New Haven; 1993 ).
Laskowski R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. J. Appl. Crystallogr. 26, 283–291 (1993).
Acknowledgements
This work was supported by grants from the National Institutes of Health (to D.J.W.), the American Cancer Society (to D.J.W.), and SRIS/DRIF funding from The University of Maryland School of Medicine and the State of Maryland (to D.J.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rust, R., Baldisseri, D. & Weber, D. Structure of the negative regulatory domain of p53 bound to S100B(ββ) . Nat Struct Mol Biol 7, 570–574 (2000). https://doi.org/10.1038/76797
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/76797
This article is cited by
-
Dynamic interactions and Ca2+-binding modulate the holdase-type chaperone activity of S100B preventing tau aggregation and seeding
Nature Communications (2021)
-
ODiNPred: comprehensive prediction of protein order and disorder
Scientific Reports (2020)
-
Computational prediction of MoRFs based on protein sequences and minimax probability machine
BMC Bioinformatics (2019)
-
Mechanisms of transcriptional regulation by p53
Cell Death & Differentiation (2018)
-
Low Energy Conformations for S100 Binding Peptide from the Negative Regulatory Domain of p53
The Protein Journal (2018)