Abstract
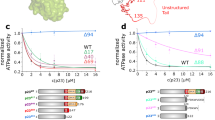
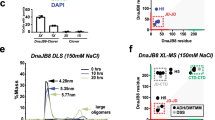
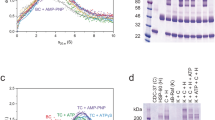
Hsp90 is a highly specific chaperone for many signal transduction proteins, including steroid hormone receptors and a broad range of protein kinases. The crystal structure of the N-terminal domain of the yeast Hsp90 reveals a dimeric structure based on a highly twisted sixteen stranded β-sheet, whose topology suggests a possible 3D-domain-swapped structure for the intact Hsp90 dimer. The opposing faces of the β-sheets in the dimer define a potential peptide-binding cleft, suggesting that the N-domain may serve as a molecular ‘clamp’ in the binding of ligand proteins to Hsp90.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wiech, H., Buchner, J., Zimmermann, R. & Jakob, U. HSP90 chaperones protein folding in vitro. Nature 358, 169–170 (1992).
Joab, I. et al. Common non-hormone binding component in non-transformed chick oviduct receptors of four natural steroids. Nature 308, 850–853 (1984).
Wilhelmsson, A. et al. The specific DMA binding activity of the dioxin receptor is modulated by the 90 kDa heat-shock protein. EMBO. J. 9, 69–76 (1990).
Opperman, H., Levinson, W. & Bishop, J.M. A cellular protein that associates with the transforming protein of Rous Sarcome Virus is also a heat-shock protein. Proc. Natl. Acad. Sci. USA 78, 1067–1071 (1981).
Cutforth, T. & Rubin, G. Mutations in Hsp83 and CDC37 impair signalling by the Seveless receptor tyrosine kinase in Drosophila. Cell 77, 1027–1036 (1994).
Aligue, R., Akhavannik, A. & Russell, P.A. A role for Hsp90 in cell-cycle control – Wee 1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 13, 6099–6106 (1994).
Stancato, L.F. et al. Raf exists in a native heterocomplex with Hsp90 and p50 that can be reconstituted in a cell-free system. J. Biol. Chem. 268, 21711–21716 (1993).
Dai, I., Kobayashi, R. & Beach, D. Physical interaction of mammalian CDC37 with CDK4. J. Biol. Chem. 271, 22030–22034 (1996).
Chen, C.F. et al. A new member of the Hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat-shock. Mol. Cell. Biol. 16, 4691–4699 (1996).
Sepehrnia, B., Paz, I.B., Dasgupta, G. & Momand, J. Heat shocked protein 84 forms a complex with mutant p53 protein predominantly within a cytoplasmic compartment of the cell. J. Biol. Chem. 271, 15084–15090 (1996).
Czar, M.J. et al. Characterisation of the protein-protein interactions determining the heat shock protein (hsp90-hsp70-hsp56) heterocomplex. J. Biol. Chem. 269, 11155–11161 (1994).
Bose, S., Weikl, T., Bügl, H. & Buchner, J. Chaperone function on Hsp90 – associated proteins. Science 274, 1715–1717 (1996).
Freeman, B.C., Toft, D.O. & Morimoto, R.I. Molecular chaperone machines: Chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science 274, 1718–1720 (1996).
Prodromou, C., Piper, P.W. & Pearl, L.H. Expression and crystallization of the yeast Hsp82 chaperone, and preliminary X-ray diffraction studies of the amino-terminal domain. Proteins Struct. Funct. Genet. 25, 517–522 (1996).
Gupta, R.S. Phylogenetic analysis of the 90 kDa heat-shock family of protein sequences and an examination of the relationships among animals, plants and fungi species. Mol. Biol. Evol. 12, 1063–1073 (1995).
Bennett, M.J., Schlunegger, M.P. & Eisenberg, D. 3D domain swapping - a mechanism for oligomer assembly. Prat. Sci. 4, 2455–2468 (1995).
Minami, Y., Kawasaki, H., Miyata, Y., Suzuki, K. & Yahara, I. Analysis of native forms and isoform compositions of the mouse 90 kDa heat-shock protein Hsp90. J. Biol. Chem. 266, 10099–10103 (1991).
Bresnick, E.H., Dalman, F.C. & Pratt, W.B. Direct stoichiometric evidence that the untransformed Mr 300,000, 9S, glucocorticoid receptor is a core unit derived from a larger heteromeric complex. Biochemistry 29, 520–527 (1990).
Minami, Y., Kimura, Y., Kawasaki, H., Suzuki, K. & Yahara, I. The carboxy terminal region of mammalian Hsp90 is required for its dimerisation and function in vivo. Mol. Cell. Biol. 14, 1459–1464 (1994)
Meng, X. et al. Mutational analysis of Hsp90a dimerisation and subcellular localisaton - dimer disruption does not impede in vivo interaction with estrogen receptor. J. Cell. Sci. 109, 1677–1687 (1996).
Smith, D.F. et al. Progesterone receptor structure and function altered by geldanamycin, an Hsp90 binding agent. Mol. Cell. Biol. 15, 6804–6812 (1995).
Kimura, Y., Yahara, I. & Lindquist, S. Role of the protein chaperone Ydj 1 in establishing Hsp90 medited signal-transduction pathways. Science 268, 1362–1365 (1993).
Boguski, M.S., Sikorski, R.S., Heiter, P. & Goebl, M. Nature 346, 114 (1990).
Smith, D.F. et al. Identification of a 60 kilodalton stress-related protein, p60, which interacts with hsp90 and hsp70. Mol. Cell. Biol. 13, 869–876 (1993).
Ratajczak, T. et al. The cyclophilin component of the unactivated estrogen receptor contains a tetratricopeptide repeat domain and shares identity with p59 (FKBP59). J. Biol. Chem. 268, 13187–13192 (1993).
Sanchez, E.R., Faber, L.E., Henzel, W.J. & Pratt, W.B. The 56–59 kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins. Biochemistry 29, 5145–5152 (1990).
Johnson, J.L. & Toft, D.O. A novel chaperone complex for steroid receptors involving heat shock protein, immunophilins and p23. J. Biol. Chem. 269, 24989–24993 (1994).
Smith, D.F. & Toft, D.O. Steroid receptors and their associated proteins. Mol. Endocrinol. 7, 4–11 (1993).
Stepanova, L., Leng, X.H., Parker, S.B. & Harper, J.W. Mammalian p50 (CDC37) is a protein kinase targetting subunit of Hsp90 that binds and stabilises CDK4. Genes Develop. 10, 1491–1502 (1996).
Brugge, J.S. Interactions of the Rous Sarcoma Virus protein pp60src with the cellular proteins pp50 and pp90. Curr. Top. Microbiol. Immunol. 123, 1–22 (1986).
Nathan, D.F. & Lindquist, S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol. Cell. Biol. 15, 3917–3925 (1995).
Miyata, Y. & Yahara, I. Interaction between casein kinase II and the 90 kDa stress protein, Hsp90. Biochemistry 34, 8123–8129 (1995).
Braig, K. et al. The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature 371, 578–586 (1994).
Sullivan, W.P. & Toft, D.O. Mutational analysis of Hsp90 binding to the progesterone receptor. J. Biol. Chem. 268, 20373–20379 (1993).
Shaknovich, R., Schue, G. & Kohtz, D.S. Conformational activation of a basic helix-loop-helix protein (MyoD1) by the C-terminal region of murine Hsp90 (Hsp84). Mol. Cell. Biol. 12, 5059–5068 (1992).
Hoffman, K. & Handschumacher, R.E. Cyclophilin-40: evidence for a dimeric complex with hsp90. Biochem. J. 307, 5–8 (1995).
Ratajczak, T. & Carrello, A. Cyclophilin-40 (Cyp-40), mapping of its Hsp90 binding domain and evidence that FKBP52 competes with Cyp-40 for Hsp90 binding. J. Biol. Chem. 271, 2961–2965 (1996).
Owens-Grillo, J.K. et al. A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J. Biol. Chem. 271, 13468–13475 (1996).
Radanyi, C., Chambraud, B. & Baulieu, E.E. The ability of the immunophilin FKBP59-HBI to interact with the 90 kDa heat-shock protein is encoded by its tetratricopeptide repeat domain. Proc. Natl. Acad. Sci. USA 91, 11197–11201 (1994).
Sikorski, R.S., Boguski, M.S., Goebl, M. & Hieter, P. A repeating amino-acid motif defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell 60, 307–317 (1990).
Hirano, T., Kinoshita, N., Morikawa, K. & Yanagida, M. Snap helix with knob and hole – essential repeats in S. pombe nuclear protein Nuc2+. Cell 60, 319–328 (1990).
Johnson, J.L. & Toft, D.O. Binding of p23 and hsp90 during assembly with the progesterone receptor. Mol. Endocrinol. 9, 670–678 (1995).
Zhu, X. et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272, 1606–1614 (1996).
Czar, M.J., Welsh, M.J. & Pratt, W.B. Immunofluorescence localization of the 90 kDa heat-shock protein to cytoskeleton. Eur. J. Cell. Biol. 70, 322–330 (1996).
Leslie, A.G.W. ‘MOSFLM Users Guide’ Cambridge, U.K., MRC-LMB.
Collaborative Computational Project No. 4 (1994) Acta Crystallogr. D50, 760–763 (1995).
Brünger, A. ‘X-PLOR Version 3.1. A system for X-ray Crystallography and NMR’, (Yale University Press, New Haven, CT, USA, 1992).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK - a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–290 (1993).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models Acta. Crystallogr. A47, 110–119 (1991).
Kraulis, P.J. MOLSCRIPT - a program to produce both detailed and schematic plots of prortein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merrit, E.A. & Murphy, M.E.P. Raster3D Version 2.0 – a program for photorealistic molecular graphics. Acta. Crystallogr. 50, 869–873 (1994).
Laskowski, R.A. SURFNET - A program for visualising molecular surfaces, cavities and intermolecular interactions. J. Mol. Graph. 13, 323–330 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prodromou, C., Roe, S., Piper, P. et al. A molecular clamp in the crystal structure of the N-terminal domain of the yeast Hsp90 chaperone. Nat Struct Mol Biol 4, 477–482 (1997). https://doi.org/10.1038/nsb0697-477
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0697-477