Abstract
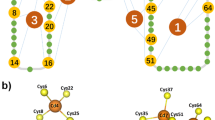
The trimeric parallel β-coil P22 tailspike contains eight cysteines per chain, but lacks disulphide bonds in the native state, in both the crystalline and solution forms. However, cysteines in a folding intermediate are reactive with thiol blocking reagents, which prevent further productive folding both in vivo and in vitro. The in vivo refolding yield was independent of the availability of metal ions, but was sensitive to redox potential. Isolation by nondenaturing gel electrophoresis of the protrimer intermediate, a trimeric folding intermediate that precedes the fully folded trimer in the in vivo and in vitro pathways, revealed the presence of interchain disulphide bonds. Incubation of the isolated protrimer with reducing agents generated the native trimer. The formation of β-sheets with interdigitated strands from different subunits in the native trimer may require the transient disulphide bonds for proper alignment. To our knowledge this is the first report of a disulphide bond present in a folding intermediate of a non-disulphide bonded protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Creighton, T.E. Proteins. Structures and Molecular Properties. 2nd ed. (W.H. Freeman and Company, New York, 1993).
Braakman, I., Hoover-Litty, H., Wagner, K.R., & Helenius, A. Folding of influenza hemagglutinin intheendoplasmicreticulum. J. Cell Biol. 114, 401–411 (1991).
Creighton, T.E. Conformation restrictions on the pathway of folding and unfolding of the pancreatic trypsin inhibitor. J. Mol. Biol., 113, 275–293 (1977).
Creighton, T.E. Effects of urea and guanidine-HCI on the folding and unfolding of pancreatic trypsin inhibitor. J. Mol. Biol. 113, 313–328 (1977).
Creighton, T.E. & Goldenberg, D.P. Kinetic role of a meta-stable native-like two-disulphide species in the folding transition of bovine pancreatic trypsin inhibitor. J. Mol. Biol. 179, 497–526 (1984).
Weissman, J.S. & Kim, P.S. The pro region of BPTI facilitates folding. Cell 71, 841–851 (1992).
Weissman, J.S. & Kim, P.S. Reexamination of the folding of BPTI: Predominance of native intermediates. Science 253, 1386–1393 (1991).
Fahey, R.C., Hunt, J.S. & Windham, G.C. On the cysteine and cystine content of proteins: Differences between intracellular and extracellular proteins. J. Mol. Evol. 10, 155–160 (1977).
Gilbert, H.F. Molecular and cellular aspects of thiol-disulfide exchange. in Advances in Enzymology (ed. Meister, A.) 69–173 (Wiley, New York, 1990).
Grubmeyer, C.T. & Gray, W.R. A cysteine residue (cysteine-116) in the histidinol binding site of histidinol dehydrogenase. Biochemistry 25, 4778–4784 (1986).
Sauer, R.T., Krovatin, W., Poteete, A.R., & Berget, P.B. Phage P22 tail protein: Gene and amino acid sequence. Biochemistry 21, 5811–5815 (1982).
Steinbacher, S. et al. Crystal structure of P22 tailspike protein: Interdigitated subunits in a thermostable trimer. Science 265, 383–386 (1994).
Yoder, M.D., Keen, N.T., & Jurnak, R. New domain motif: The structure of pectate lyase C, a secreted plant virulence factor. Science 260, 1503–1507 (1993).
Baumann, U., Wu, S., Flaherty, K.M., & McKay, D.B. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 12, 3357–3364 (1993).
Goldenberg, D., Berget, P., & King, J. Maturation of the tailspike endrorhamnoside of Salmonella phage P22. J. Biol. Chem. 257, 7864–7871 (1982).
Goldenberg, D. & King, J. Trimeric intermediate in the in vivo folding and subunit assembly of the tail spike endorhamnosidase of bacteriophage P22. Proc. Natl. Acad. Sci. USA 79, 3403–3407 (1982).
Haase-Pettingell, C.A. & King, J. Formation of aggregates from a thermolabile in-vivo folding intermediate in P22 tailspike maturation a model for inclusion body formation. J. Biol. Chem. 263, 4977–4983 (1988).
Seckler, R., Fuchs, A., King, J. & Jaenicke, R. Reconstitution of the thermostable trimeric phage P22 tailspike protein from denatured chains in vitro. J. Biol. Chem. 264, 11750–11753 (1989).
Fuchs, A., Seiderer, C. & Seckler, R. In vitro folding pathway of phage P22 tailspike protein. Biochemistry 30, 6598–6604 (1991).
Speed, M.A., Wang, D.I.C. & King, J. Multimeric intermediates in the pathway to the aggregated inclusion body state for P22 tailspike polypeptide chains. Prot. Sci. 4, 900–908 (1995).
Danner, M. & Seckler, R. Mechanism of phage P22 tailspike protein folding mutations. Prot. Sci. 2, 1869–1881 (1993).
Goldenberg, D.P., Smith, D.H. & King, J. Genetic analysis of the folding pathway for the tail spike of phage P22. Proc. Natl. Acad. Sci. USA 80, 7060–7064 (1983).
Sargent, D., Benevides, J.M., Yu, M.-H., King, J. & Thomas Jr., G.J.T. Secondary structure and thermostability of the phage P22 tailspike: Analysis of raman spectroscopy of the wild type protein and a temperature-sensitive folding mutant. J. Mol. Biol. 199, 491–502 (1988).
Sather, S.K. & King, J. Intracellular trapping of a cytoplasmic folding intermediate of the phage P22 tailspike using iodoacetamide. J. Biol. Chem. 269, 25268–25276 (1994).
Lippard, S.J. & Berg, J.M. Principles of Bioinorganic Chemistry. (University Science Books, Mill Valley, California, 1994).
Hwang, C., Sinskey, A.J. & Lodish, H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257, 1496–1502 (1992).
Gilbert, H.F. The formation of native disulfide bonds. in Mechanisms of Protein Folding (ed. Pain, R.H.) 104–136 (IRL Press, New York, 1994).
Derman, A.I., Prinz, W.A., Belin, D. & Beckwith, J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science 262, 1744–1747 (1993).
Freedman, R.B. Protein folding in the cell in Protein Folding (ed. Creighton, T.E.) 455–539 (W.H. Freeman and Company, New York, 1992).
Winston, R., Botstein, D. & Miller, J. Characterization of amber and ochre suppressors in Salmonella typhimurium. J. Bacteriol. 137, 433–439 (1979).
King, J. & Yu, M.-H. Mutational analysis of protein folding pathway using the P22 tailspike endorhamnosidase in Methods in Enzymology (eds Hirs, C.W.H. & Timashiff, S.) 250–266 (Academic Press, New York, 1986).
Yamamoto, K.R., Alberts, B.M., Benzinger, B., Lawhorne, L., & Treiber, G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 40, 734–744 (1970).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning, A Laboratory Manual. (Cold Spring Harbor Laboratory Press, New York, 1989).
Kirley, T.L. Reduction and fluorescent labeling of cysteine-containing proteins for subsequent structural analyses. Anal. Biochem. 180, 231–236 (1989).
Koradi, R., Billeter, M. & Wüthrich, K. MOLMOL: a program for display and analysis of macromolecular structures. J. Mol. Graph. 14, 51–55 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robinson, A., King, J. Disulphide-bonded intermediate on the folding and assembly pathway of a non-disulphide bonded protein. Nat Struct Mol Biol 4, 450–455 (1997). https://doi.org/10.1038/nsb0697-450
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0697-450
This article is cited by
-
A genetic analysis of an important hydrophobic interaction at the P22 tailspike protein N-terminal domain
Archives of Virology (2018)
-
Characterization of Capsicum annuum Recombinant α- and β-Tubulin
Applied Biochemistry and Biotechnology (2010)
-
Nature's transitory covalent bond
Nature Structural Biology (1997)