Abstract
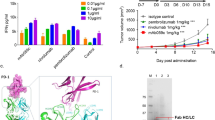
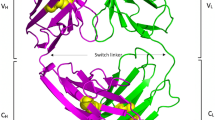
The crystal structures of the murine BR96 Fab and its human chimera have been determined in complex with the nonoate methyl ester derivative of Lewis Y (nLey) at 2.8 Å and 2.5 Å resolution, respectively. BR96 binds the carbohydrate in a large pocket which is formed by residues of all CDR loops except L2. The binding of the carbohydrate is mediated predominantly by aromatic residues in BR96. Analysis of the structure suggests that BR96 is capable of recognizing a structure larger than the Ley tetrasaccharide, providing a possible explanation for its high tumour selectivity. The structure provides a rationale for mutagenesis experiments that have resulted in BR96 CDR loop mutants with increased affinity for nLey and/or tumour cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hellström, K.E. & Hellström, I. Principles of tumour immunity: tumour antigens. In Biologic Therapy of Cancer (Eds, DeVita, V.T., Jr, Hellman, S., & Rosenberg, S.A.) 35–52. (J.B. Lippincott Co., Philadelphia; 1991).
Trail, P.A. et al. Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Science 261, 212–215 (1993).
Hellström, I., Garrigues, H.J., Garrigues, U. & Hellström, K.E. Highly tumour-reactive, internalizing, mouse monoclonal antibodies to Ley-related cell surface antigens. Cancer Res. 50, 2183–2190 (1990).
Hakomori, S. Aberrant glycosylation in tumours and tumour-associated carbohydrate antigens. Advan. Cancer Res. 52, 257–331 (1989).
Garrigues, J., Anderson, J., Hellström, K.E., Hellström, I., Mab BR96 blocks cell migration and binds to lysosomal membrane glycoprotein on cell surface microspikes and ruffled membranes. J. cell. Biol. 125, 129–142 (1994).
Garrigues, J., Garrigues, U., Hellström, I. & Hellström, K.E. Ley specific antibody with potent anti-tumour activity is internalized and degraded in lysosomes. Am. J. Pathol. 142, 607–622 (1993).
Willner, D. et al. (6-Maleimidocaproyl)hydrazone of doxorubicin–a new derivative for the preparation of immunoconjugates of doxorubicin. Bioconjugate Chem. 4, 521–527 (1993).
Yelton, D.E. et al. Affinity maturation of the BR96 anticarcinoma antibody in vitro by codon-based mutagenesis. J. Immunol. in the press.
Bajorath, J. Three-dimensional model of the BR96 monoclonal antibody variable fragment. Bioconjugate Chem. 5, 213–219 (1994).
Chang, C.Y., Jeffrey, P.D., Bajorath, J., Hellström, I., Hellström, K.E. & Sheriff, S. Crystallization and preliminary X-ray analysis of the monoclonal anti-tumour antibody BR96 and its complex with the Lewis Y determinant. J. molec. Biol. 235, 372–376 (1994).
Sheriff, S. Some methods for examining the interactions between two molecules. ImmunoMethods 3, 191–196 (1993).
Connolly, M.L. Analytical molecular surface calculation. J. appl. Crystallogr. 16, 548–558 (1983).
Novotny, J., Bruccoleri, R.E. & Saul, F.A. On the attribution of binding energy in antigen—antibody complexes McPC 603, D1.3, and HyHEL-5. Biochemistry 28, 4735–4749 (1989).
Pastan, I., Lovelace, E.T., Gallo, M.G., Rutherford, A.V., Magnani, J.L. & Willingham, M.C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 51, 3781–3787 (1991).
Kaneko, T. et al. Preparation of mouse-human chimeric antibody to an embryonic carbohydrate antigen, Lewis Y. J. Biochem, Tokyo 113, 114–117 (1993).
Cygler, M., Rose, D.R. & Bundle, D.R. Recognition of a cell-surface oligosaccharide of pathogenic Salmonella by an antibody Fab fragment. Science 253, 442–445 (1991).
Bundle, D.R. et al. Molecular recognition of a Salmonella trisaccharide epitope by monoclonal antibody Se155-4. Biochemistry 33, 5172–5182 (1994).
Bundle, D.R., Baumann, H., Brisson, J.-R., Gagné, S.M., Zdanov, A. & Cygler, M. Solution structure of a trisaccharide-antibody complex: comparison of NMR measurements with a crystal structure. Biochemistry 33, 5183–5192 (1994).
Padlan, E.A. & Kabat, E.A. Modeling of antibody combining sites. Meths Enzymol. 203, 3–21 (1991).
Quiocho, F.A. Carbohydrate-binding proteins: tertiary structures and protein-sugar interactions. A. Rev. Biochem. 55, 287–315 (1986).
Vyas, N.K. Atomic features of protein-carbohydrate interactions. Curr. Opin. Struct. Biol. 1, 732–740 (1991).
Bundle, D.R. & Young, N.M. Carbohydrate—protein interactions in antibodies and lectins. Curr. Opin. struct. Biol. 2, 666–673 (1992).
Padlan, E.A. On the nature of antibody combining sites: unusual structural features that may confer on these site an enhanced capacity for binding ligands. Proteins Struct. Funct. Genet. 7, 112–124 (1990).
Chothia, C. et al. The conformations of immunoglobulin hypervariable regions. Nature 342, 877–883 (1989).
Fitzgerald, P.M.D. MERLOT, an integrated package of computer programs for the determination of crystal structures by molecular replacement. J. appl. Crystallogr. 21, 273–278 (1988).
Fujinaga, M. & Read, R.J. Experiences with a new translation-function program. J. appl. Crystallogr. 20, 517–521 (1987).
Brünger, A.T. Solution of a fab (26-10)/digoxin complex by generalized molecular replacement. Acta crystallogr. A 47, 195–204 (1991).
Brünger, A.T. X-PLOR version 3.1: A system for X-ray crystallography and NMR. Yale University Press, New Haven (1992).
Rini, J.M., Schulze-Gahmen, U. & Wilson, I.A. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science 255, 959–965 (1992).
Jeffrey, P.D., Schildbach, J.F., Chang, C.Y., Kussie, P., Margolies, M.N. & Sheriff, S. Structure and specificity of the anti-digoxin antibody 40-50. J. molec. Biol., 248, 344–360 (1995).
Stanfield, R.L., Fieser, T.M., Lerner, R.A. & Wilson, L.A. Crystal structures of an antibody to a peptide and its complex with peptide antigen at 2.8 Å. Science 248, 712–719 (1990).
Cygler, M. & Anderson, W.F. Application of the molecular replacement method to multidomain proteins.1. determination of the orientation of an immunoglobulin Fab fragment. Acta crystallogr. A44, 38–45 (1988).
Sack, J.S. CHAIN—a crystallographic modeling program. J. molec. Graph. 6, 224–225 (1988).
Engh, R.A. & Huber, R. Accurate bond and angle parameters for X-ray protein structure refinement. Acta crystallogr. A 47, 392–400 (1991).
Richardson, J.S. The anatomy and taxonomy of protein structure. Adv. prot. Chem. 34, 167–339 (1981).
Gelin, B.R. & Karplus, M. Side-chain torsional potentials: effect of dipeptide, protein and solvent environment. Biochemistry 18, 1256–1268 (1979).
Luzzati, V. Traitement statistique des erreurs dans la determination des structures cristallines. Acta crystallogr. 5, 802–810 (1952).
Kabat, E.A., Wu, T.T., Perry, H.M., Gottesman, K.S. & Foeller, C. Sequences of proteins of immunological interest (National Institutes of Health, Bethesda, MD) (1991).
Carson, M. Ribbons 2.0. J. appl. Crystallogr. 24, 958–961 (1991).
Nicholls, A., Sharp, K.A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jeffrey, P., Bajorath, J., Chang, C. et al. The X-ray structure of an anti-tumour antibody in complex with antigen. Nat Struct Mol Biol 2, 466–471 (1995). https://doi.org/10.1038/nsb0695-466
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0695-466
This article is cited by
-
Carbohydrate residues downstream of the terminal Galα(1,3)Gal epitope modulate the specificity of xenoreactive antibodies
Immunology & Cell Biology (2007)
-
Germline antibody recognition of distinct carbohydrate epitopes
Nature Structural & Molecular Biology (2003)
-
Bands without Rock and Roll
Nature Structural Biology (1996)
-
A Trojan horse with a sweet tooth
Nature Structural & Molecular Biology (1995)