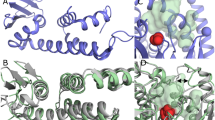
Abstract
It has long been proposed that the higher activity of phospholipase A2 (PLA2) for substrates presented as multimolecular aggregates compared to dispersed molecules (interfacial activation) arises due to a conformational change in the enzyme. X-ray studies have, however, failed to identify any such change. Here we report the solution structures of porcine pancreatic PLA2 both free and as a ternary complex with micelles and a competitive inhibitor. Important differences between these structures indicate that conformational changes may play an important role in the mechanism of interfacial activation in PLA2s.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Dennis, E.A. Phospholipase A2 mechanism: Inhibition and role in arachidonic acid release. Drug. Dev. Res. 10, 205–220 (1987).
Irvine, R.F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem. J. 204, 3–16 (1982).
Dennis, E.A. Regulation of eicosanoid production: role of phospholipases and inhibitors. Biotech. 5, 1294–1300 (1987).
Snyder, F. Chemical and biochemical aspects or “platelet activating factor”, a novel class of actylated ether-linked choline-phospholipids. Med. Res. Rev. 5, 107–140 (1985).
Verger, R. & de Haas, G. H. Interfacial enzyme kinetics of lipolysis. A. Rev. Biophys. Bioeng. 5, 77–119 (1976).
Volwerk, J.J. & de Haas, G. H., Molecular Biology of Lipid-proteininteractions (eds. Griffith, O.H. & Jost, P.C.) 69–149 (Wiley, New York; 1982).
Dijkstra, B.W., Renetseder, R., Kalk, K.H., Hol, W.G.J. & Drenth,J. Structure of porcine pancreatic phospholipase A2 at 2.6 Å resolution and comparison with bovine phospholipase A2 . J. molec. Biol. 168, 163–179 (1983).
Thunnissen, M.M.G.M. et al. X-ray structure of phospholipase A2 complexed with a substrate-derived inhibitor. Nature 374, 689–691 (1990).
Scott, D.L., White, S.P., Otwinowski, Z., Yuan, K., Gelb, M.H. & Sigler, P.B. Interfacial catalysis: the mechanism of phospholipase A2 . Science 250, 1541–1546 (1990).
Scott, D.L., Otwinowski, Z., Gelb, M.H. & Sigler, P.B. Crystal structure of a bee-venom phospholipase A2 in a complex with a transition-state analogue. Science 250, 1563–1566 (1990).
White, S.P., Scott, D.L., Otwinowski, Z., Gelb, M.H. & Sigler, P.B. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science 250, 1560–1563 (1990).
Yu, L. & Dennis, E.A. Critical role of a hydrogen bond in the interaction of phospholipase A2 with transition-state and substrate analogues. Proc. natn. Acad. Sci. U.S.A. 88, 9325–9329 (1991).
Finzel, B.C., Ohlendorf, D.H., Weber, P.C. & Salemme, F.R. independent crystallographic refinement of porcine pancreatic phospholipase A2 at 2.4 Å resolution. Acta crystallogr. B47, 558–559 (1991).
Brunie, S., Bolin, J., Gewirth, D. & Sigler, P.B. The refined crystal structure of dimeric phospholipase A2 at 2.5 Å. J. biol. Chem. 260, 9742 9749 (1985).
Renetseder, R., Brunie, S., Dijkstra, B.W., Drenth, J. & Sigler, P.B. A comparison of the crystal structures of phospholipase A2 from bovine pancreas and Crotalus atrox snake venom.J. biol. Chem. 260, 11627–11634 (1985).
Dijkstra, B.W., Kalk, K.H., Hoi, W.G.J. & Drenth, J. tructure of bovine pancreatic phospholipase A2 at 1.7 Å resolution. J. molec. Biol. 147, 97–123 (1981).
Dijkstra, B.W., van Nes, G.J.H., Kalk, K.H., Brandenburg, N.P., Hol, W.G.J. & Drenth, J. The structure of bovine prophospholipase A2 at 3.0 Å resolution. Acta crystallogr. B38, 793–799 (1982).
Dijkstra, B.W., Kalk, K.H., Drenth, J., de Haas, G H., Egmond, M.R., & Slotboom, A.J. Role of the N-terminus in the interaction of pancreatic phospholipase A2 with aggregated substrates. Properties and crystal structure of transaminated phospholipase A2 . Biochemistry 23, 2759–2766 (1984).
Slotboom, A.J. & de Haas, G H. Specific transformations at the N-terminal region of phospholipase A2 . Biochemistry 14, 5394–5399 (1975).
Verheij, H.M., Egmond, M.R. & de Haas, G. H. Chemical modification of the α-amino group in snake venom phospholipases A2 . Biochemistry 20, 94–99 (1981).
Peters, A.R. et al. Conformational changes in phospholipase A2 upon binding to micellar interfaces in the absence and presence of competitive inhibitors. A1H and 15N NMR study. Biochemistry 31, 10024–10030 (1992).
Sarda, L. & Desnuelle, P. Action de la lipase pancreatique sur les esters en emulsion. Biochim. biophys. Acta 30, 513–520 (1958).
Brady, I. et al. A serine protease triad forms the catalytic centre of a triacylglycerol lipase. Nature 343, 767–770 (1990).
Brzozowski, A.M. et al. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature 351, 491–494 (1991).
van Tilbeurgh, H., Egloff, M.-P., Martinez, C., Rugani, N., Verger, R. & Cambillau, C. Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by X-ray crystallography. Nature 362, 814–820 (1993).
Senn, H., Werner, B., Messerle, B.A., Weber, C., Traber, R. & Wuthrich, K. Stereospecific assignment of the methyl 1H NMR lines of valine and leucine in polypeptides by nonrandom 13C labelling. FEBS Letts 249, 113–118 (1989).
Kay, L.E. & Bax, A. New methods for the measurement of NH-CαH coupling constants in 15N-labeled proteins. J. magn. Res. 86, 110–126 (1989).
Pardi, A., Billeter, M. & Wüthrich, K. Calibration of the angular dependence of the amide proton-Cα proton coupling constants, 3JHNα, in a globular protein. J. molec. Biol. 180, 741–747 (1984).
Havel, T.F. An evaluation of computational strategies for use in the determination of protein structure from distance constraints obtained by nuclear magnetic resonance. Prog. Biophys. molec. Biol. 56, 43–78 (1991).
de Haas, G.H., Dijkman, R., Ransac, S. & Verger, R. Competitive inhibition of lipolytic enzymes . IV. Structural details of acylamino phospholipid analogues important for the potent inhibitory effects on pancreatic phospholipase A2 . Biochim. biophys. Acta 1046, 249–257 (1990).
Kraulis, P.J. Molscript: a program to produce both detailed and schematic plots for protein structures. J. appl. Crystallogr. 24, 946–950 (1991).
Morris, A.L., MacArthur, M.W., Hutchinson, G.E. & Thornton, J.M. Stereochemical quality of protein structure coordinates. Proteins 12, 345–364 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
den Berg, B., Tessari, M., Boelens, R. et al. NMR structures of phospholipase A2 reveal conformational changes during interfacial activation. Nat Struct Mol Biol 2, 402–406 (1995). https://doi.org/10.1038/nsb0595-402
Issue Date:
DOI: https://doi.org/10.1038/nsb0595-402
This article is cited by
-
Active site competition is the mechanism for the inhibition of lipoprotein-associated phospholipase A2 by detergent micelles or lipoproteins and for the efficacy reduction of darapladib
Scientific Reports (2020)
-
Structural aspects and activation mechanism of human secretory group IIA phospholipase
European Biophysics Journal (2020)
-
A twist in the tale of lipolytic enzymes
Nature Structural & Molecular Biology (1995)