Abstract
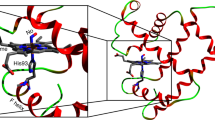
The nature of ligand motion within proteins has been investigated by measuring femtosecond time-resolved infrared (IR) spectra of CO photodissociated from the haem of myoglobin. Upon dissociation, the CO rotates approximately 90° and becomes trapped within a ligand docking site located near the binding site. Two trajectories, distinguished spectroscopically and kinetically with time constants of 0.20±0.05 ps and 0.52±0.10 ps, lead to CO located within the docking site with opposite orientations. The protein reorganizes about the ‘docked’ CO with a time constant of 1.6±0.3 ps and quickly establishes an energetic barrier that inhibits the reverse rebinding process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Loyd, C.R., Eyring, E.M. & Ellis, J.W.R. Uptake and release of O2 by myohemerythrin. Evidence for different rate-determining steps and a caveat. J. Am. Chem. Soc. 117, 11993–11994 (1995).
Springer, B.A., Sligar, S.G., Olson, J.S. & Phillips, G.N., Jr. Mechanisms of ligand recognition in myoglobin. Chem. Rev. 94, 699–714 (1994).
Verma, A., Hirsch, D.J., Glatt, C.E., Ronnett, G.V. & Snyder, S.H. Carbon monoxide: a putatitive neural messenger. Science 259, 381–384 (1993).
Snyder, S.H. Nitric oxide: first in a new class of neurotransmitters? Science 257, 494–496 (1992).
Antonini, E. & Brunori, M. Hemoglobin and Myoglobin in Their Reactions With Ligands, (North-Holland Publishing Company, London, 1971).
Collman, J.P., Brauman, J.I., Halbert, T.R. & Suslick, K.S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc. Natl. Acad. Sci. U. S. A. 73, 3333–3337 (1976).
Swiss-Prot Protein Database. (National Center of Biotechnology Information, Bethesda, 1994).
Lim, M., Jackson, T.A. & Anfinrud, P.A. Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe-C-O. Science 269, 962–966 (1995).
Lim, M., Jackson, T.A. & Anfinrud, P.A. Mid-IR vibrational spectrum of CO after photodissociation from heme: evidence for a ligand docking site in the heme pocket of hemoglobin and myoglobin. J. Chem. Phys. 102, 4355–4366 (1995).
Rothberg, L., Jedju, T.M. & Austin, R.H. Ligand dynamics in the photodissociation of carboxyhemoglobin by subpicosecond transient infrared spectroscopy. Biophys. J. 57, 369–373 (1990).
Anfinrud, P.A., Han, C. & Hochstrasser, R.M. Direct observations of ligand dynamics in hemoglobin by subpicosecond infrared spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 86, 8387–8391 (1989).
Eaton, W.A. & Hofrichter, J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 76, 175–261 (1981).
Alben, J.O. et al. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc. Natl. Acad. Sci. U. S. A. 79, 3744–3748 (1982).
Schlichting, I., Berendzen, J., Phillips, G.N., Jr. & Sweet, R.M. Crystal structure of photolysed carbonmonoxy-myoglobin. Nature 371, 808–812 (1994).
Teng, T.Y., Srajer, V. & Moffat, K. Photolysis-induced structural changes in single crystals of carbonmonoxy myoglobin at 40 K. Nature Struct. Biol. 1, 701–705 (1994).
Hartmann, H. et al. X-ray structure determination of a metastable state of carbonmonoxy myoglobin after photodissociation. Proc. Natl. Acad. Sci. USA 93, 7013–7016 (1996).
Chance, M.R. et al. Global mapping of structural solutions provided by the extended x-ray absorption fine structure ab initio code FEFF 6.01: Structure of the cryogenic photoproduct of the myoglobin-carbon monoxide complex. Biochemistry 35, 9014–9023 (1996).
Case, D.E. & Karplus, M. Dynamics of ligand binding to heme proteins. J. Mol. Biol. 132, 343–368 (1979).
Elber, R. & Karplus, M. Enhanced sampling in molecular dynamics: use of the time-dependent Hartree approximation for a simulation of carbon monoxide diffusion through myoglobin. J. Am. Chem. Soc. 112, 9161–9175 (1990).
Austin, R.H., Beeson, K.W., Eisenstein, L., Frauenfelder, H. & Gunsalus, I.C. Dynamics of ligand binding to myoglobin. Biochemistry 14, 5355–5373 (1975).
Altman, P.L. & Dittmer, D.S. Respiration and Circulation. in Biological Handbooks (Federation of American Societies for Experimental Biology, Bethesda, 1971).
Alben, J.O. et al. Isotope effect in molecular tunneling. Phys. Rev. Lett. 44, 1157–1163 (1980).
Alberding, N. et al. Tunneling in ligand binding to heme proteins. Science 192, 1002–1004 (1976).
Lim, M., Jackson, T.A. & Anfinrud, P.A. Femtosecond near-IR absorbance study of photoexcited myoglobin: the dynamics of electronic and thermal relaxation. J. Phys. Chem. 100, 12043–12051 (1996).
Henry, E.R., Eaton, W.A. & Hochstrasser, R.M. Molecular dynamics simulations of cooling in laser-excited heme proteins. Proc. Natl. Acad. Sci. U. S. A. 83, 8982–8986 (1986).
Tian, W.D., Sage, J.T. & Champion, P.M. Investigations of ligand association and dissociation rates in the “open” and “closed” states of myoglobin. J. Mol. Biol. 233, 155–166 (1993).
Bourgeois, D. et al. Feasability and realization of single-pulse Laue diffraction on macromolecular crystals at ESRF. J. Synchrotron Radiat. 3, 65–74 (1996).
Srajer, V. et al. Photolysis of the carbon monoxide complex of myoglobin: Nanosecond time-resolved crystallography. Science 274, 1726–1729 (1996).
Anfinrud, P.A., Lim, M. & Jackson, T.A. Femtosecond IR spectroscopy: methods and applications to protein dynamics. Proc. SPIE-Int. Soc. Opt. Eng. (Longer Wavelength Lasers and Applications) 2138, 107–115 (1994).
Moore, J.N., Hansen, P.A. & Hochstrasser, R.M. A new method for picosecond time-resolved infrared spectroscopy: applications to CO photodissociation from iron porphyrins. Chem. Phys. Lett. 138, 110–114 (1987).
Moore, J.N., Hansen, P.A. & Hochstrasser, R.M. Iron-carbonyl bond geometries of carboxymyoglobin and carboxyhemoglobin in solution determined by picosecond time-resolved infrared spectroscopy. Proc. Natal. Acad. Sci. U. S. A. 85, 5062–5066 (1988).
Hansen, P.A., Moore, J.N. & Hochstrasser, R.M. Determination of the iron-carbonyl bond geometry of carboxyprotoheme in solution using picosecond infrared-optical photoselection. Chem. Phys. 131, 49–62 (1989).
Locke, B., Lian, T. & Hochstrasser, R.M. Determination of Fe-CO geometry and heme rigidity in carbonmonoxyhemoglobin using femtosecond IR spectroscopy. Chem. Phys. 158, 409–419 (1991).
Lian, T., Locke, B., Kitagawa, T., Nagai, M. & Hochstrasser, R.M. Determination of Fe-CO geometry in the subunits of carbonmonoxy hemoglobin m boston using femtosecond infrared spectroscopy. Biochemistry 32, 5809–5814 (1993).
Locke, B., Lian, T. & Hochstrasser, R.M. Erratum of Chemical Physics 158 (1991) 409-419. Chem. Phys. 190, 155 (1995).
Ormos, P. et al. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc. Natl. Acad. Sci. U. S. A. 85, 8492–8496 (1988).
Braunstein, D.P. et al. Ligand binding to heme proteins: III. FTIR studies of His-E7 and Val-E11 mutants of carbonmonoxymyoglobin. Biophys. J. 65, 2447–2454 (1993).
Rothberg, L.J., Roberson, M. & Jedju, T.M. Protein dynamics at physiological temperature. Proc. SPIE-Int. Soc. Opt. Eng. 1599, 309–315 (1991).
Straub, J.E., Karplus, M. Molecular dynamics study of the photodissociation of carbon monxide from myoglobin: ligand dynamics in the first 10ps. Chem. Phys. 158, 221(1991).
Vitkup, D. Petsko, G.A. & Karplus, M. Dissociated Co in myoglobin: comparison between molecular dynamics and X-ray results. Nat. Struct. Biol. 4, 200–206 (1997).
Ma, J., Huo, S. & Straub, J.E. Molecular dynamics simulations study of the B-states of solvated carbonmonoxymyoglobin, (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lim, M., Jackson, T. & Anfinrud, P. Ultrafast rotation and trapping of carbon monoxide dissociated from myoglobin. Nat Struct Mol Biol 4, 209–214 (1997). https://doi.org/10.1038/nsb0397-209
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0397-209
This article is cited by
-
Experimental demonstration of the novel “van-Hove integral method (vHI)” for measuring diffusive dynamics by elastic neutron scattering
Scientific Reports (2021)
-
Short-lived metal-centered excited state initiates iron-methionine photodissociation in ferrous cytochrome c
Nature Communications (2021)
-
A Quantitative Comparison of the Counting Significance of van Hove Integral Spectroscopy and Quasielastic Neutron Scattering
Scientific Reports (2020)
-
The Impact of Electron Correlation on Describing QM/MM Interactions in the Attendant Molecular Dynamics Simulations of CO in Myoglobin
Scientific Reports (2020)
-
Elastic Scattering Spectroscopy (ESS): an Instrument-Concept for Dynamics of Complex (Bio-) Systems From Elastic Neutron Scattering
Scientific Reports (2016)