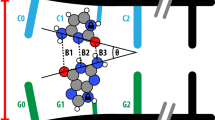
Abstract
One– and two–dimensional NMR shows that the carcinogen 2–aminofluorene exists in two unique, interchangeable conformations when covalently bound to a model human c–H–ras1 proto–oncogene codon 61 oligomer duplex. In one conformation the 2–aminofluorene moiety protrudes out of the major groove leaving the Watson–Crick base pairing of the cytosine and 2–aminofluorene–guanine bases intact, consistent with the ability of replicating enzymes to bypass the lesion and correctly incorporate cytosine. The second form of the modified oligomer duplex may be representative of a pre–mutagenic conformation in that the 2–aminofluorene moiety is stacked within the DNA helix, disrupting base pairing between the 2–aminofluorene–modified guanine and its complementary cytosine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kriek, E. Fifty years of research on N-acetyl-2-aminofluorene, one of the most versatile compounds in experimental cancer research. J. Cancer Res. clin. Oncol. 118, 481–489 (1992).
Miller, J.A. Carcinogenesis by chemicals: An overview — G. H. A. Clowes Memorial Lecture. Cancer Res. 30, 559–576 (1970).
Beland, F.A. & Kadlubar, F.F. in Handbook of Experimental Pharmacology 94/1, 281–285 (eds Cooper, C.S. & Grover, P. L.) (Springer-Verlag, Heidelberg, 1990).
Kriek, E., Miller, J.A., Juhl, U. & Miller, E.C. 8-(N-2-Flurenoacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry 6, 177–182 (1967).
Irving, C.C. Enzymatic deacetylation of N-hydroxy-2-acetylaminofluorene by liver microsomes Cancer Research 26, 1390–1396 (1966).
Beland, F.A., Dooley, K.L. & Jackson, C.D. Persistance of DNA adducts in rat liver and kidney after multiple doses of the carcinogen N-hydroxy-2-acetylaminofluorene. Cancer Res. 42, 1348–1354 (1982).
Gupta, R.C. & Dighe, N.R. Formation and removal of DNA adducts in rat liver treated with N-hydroxy derivatives of 2-acetylaminofluorene, 4-acetylaminobiphenyl, and 2-acetylaminophenanthrene. Carcinogenesis 5, 343–349 (1984).
Poirier, M.C., Fullerton, N.F., Kinouchi, T., Smith, B.A. & Beland, F. A. Comparison between DNA adduct formation and tumorigenesis in livers and bladders of mice chronically fed 2-acetylaminofluorene. Carcinogenesis 12, 895–900 (1991).
Gupta, P.K., Lee, M. & King, C.M. Comparison of mutagenesis induced in single- and double-stranded M13 viral DNA by treatment with N-hydroxy-2-aminofluorene. Carcinogenesis 9, 1337–1345(1988)
Carothers, A.D. et al A mutational hot spot induced by N-hydroxy-aminofluorene in dihydrofolate reductase mutants of Chinese hamster ovary cells. Carcinogenesis 14, 2181–2184 (1993).
Mah, M.C., Maher, V.M., Thomas, H., Reid, T.M., King, C.M. & McCormick, J.J. Mutations induced by aminofluorene–DNA adducts during replication in human cells. Carcinogenesis 10, 2321–2328(1989).
Bichara, M. & Fuchs, R.P.P. DNA Binding and mutation spectra of the carcinogen N-2-aminofluorene in Escherichia coli: A correlation between the conformation of the premutagenic lesion and the mutagenic specificity. J. molec. Biol. 183, 341–351 (1985).
Shibutani, S. & Grollman, A.P. On the mechanism of frameshift (deletion) mutagenesis in vitro. J. biol. Chem. 268, 11703–11710 (1993).
Michaels, M.L., Reid, T.M., King, C.M. & Romano, L.J. Accurate in vitro translesion synthesis by Escherichia coli DNA polymerase I (large fragment) on a site-specific, aminofluorene-modified oligonucleotide. Carcinogenesis 12, 1641–1646 (1991).
Lutgerink, J.T. et al Bypass of the major aminofluorene-DNA adduct during in vivo replication of single- and double-stranded fX174 DNA treated with N-hydroxy-2-aminofluorene. Carcinogenesis 6, 1501–1506(1985).
Santella, R.M., Kreik, E. & Grunberger, D. Circular dichroism and proton magnetic resonance studies of dApdG modified with 2-aminofluorene and 2-acetyla-aminofluorene. Carcinogenesis 1, 897–902 (1980).
Leng, M., Ptak, M. & Rio, P. Conformation of acetylaminofluorene and aminofluorene modified guanosine and guanosine derivatives. Biochem. Biophys. Res. Comm. 96, 1095–1102(1980).
Evans, F.E., Miller, D.W. & Beland, F.A. Sensitivity of the conformation of deoxyguanosine to binding at the C-8 position by N-acetylated and unacetylated 2-aminofluorene. Carcinogenesis 1, 955–959 (1980).
Kriek, E. and Spelt, C.E. Differential excision from DNA of the C-8 deoxyguanosine reaction products of N-hydroxy-2-aminofluorene and N-acetoxy-N-acetyl-2-aminofluorene by endonuclease S1 from Aspergiilus Oryzae. Cancer Lett. 7, 147–154 (1979).
Sage, E., Spodheim-Maurizot, M., Rio, P., Leng, M. & Fuchs, R.P.P. Discrimination by antibodies between local defects in DNA induced by 2-aminofluorene derivatives. FEBS Lett. 108, 66–68 (1979).
Hingerty, B.E. & Broyde, S. Energy minimized structures of carcinogen-DNA adducts: 2-acetylaminofluorene and 2-aminofluorene. J. Biomolec. Struc. Dyn. 4, 365–372 (1986).
Broyde, S. & Hingerty, B. Conformation of 2-aminofluorene-modified DNA. Biopolymers 22, 2423–2441(1983).
Lipkowitz, K.B., Chevalier, T., Widdifield, M. & Beland, F.A. Force field conformational analysis of aminofluorene and acetylaminofluorene substituted deoxyguanosine. Chem. Biol. Interact. 40, 57–76(1982).
Norman, D. et al. NMR and computational characterization of the N-(deoxyguanosin-8-yl)aminofluorene adduct [(AF)G] opposite adenosine in DNA: (AF)G[syn]•A[anti] pair formation and its pH dependence. Biochemistry 28, 7462–7476 (1989).
van Houte, L.P.A., Westra, J.G., Retel, J. & van Grondelle, R.A. Circular dichroism study on the conformation of d(CGT) modified with N-acetyl-2-aminofluorene or 2-aminofluorene. J. Biomolec. Struct. Dyn. 9, 45–59 (1991).
Wemmer, D.E. . in Biological Magnetic Resonance. 10 (eds Berliner, L. J., & Reuben, J.) 195–264 (Plenum, New York, 1992).
Wüthrich, K. NMR of Proteins and Nucleic Acids (Wiley, New York, 1986).
Skelnár, V., Brooks, B.R., Zon, G. & Bax, A. Absorption mode two-dimensional NOE spectroscopy of exchangeable protons in oligonucleotides. FEBS Lett. 216, 249–252 (1987).
Grunberger, D. & Carothers, A. Changes in DNA conformation and types of mutation induced in CHO dhfr gene by N-2-acetylaminofluoren and N-2-aminofluorene. Collect. Czech. chem. Commun. 56, 1151–1165 (1991).
Shibutani, S., Takeshita, M. & Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged 8-oxodG. Nature 348, 431–434 (1991).
Ripley, L.S. Frameshift mutation: Determinants of specificity. A. Rev. Genet. 24, 189–213 (1990).
Kunkel, T.A. Misalignment-mediated DNA synthesis errors. Biochemistry 29, 8003–8011 (1990).
Belguise-Valladier, P. & Fuchs, R.P.P. Strong sequence-dependent polymorphism in adduct-induced DNA structure: Analysis of single N-2–acetylaminofluorene residues bound within the Nar I mutation hot spot. Biochemistry 30, 10091–10100 (1991).
Veaute, X. & Fuchs, R.P.P. Polymorphism in N-2-acetylaminofluorene induced DNA structure as revealed by DNase I footprinting. Nucl. Acids Res. 19, 5603–5606 (1991).
Garcia, A., Lambert, I.B. & Fuchs, R.P.P. DNA adduct-induced stabilization of slipped frameshift intermediates within repetitive sequences: Implications for mutagenesis. Proc. natn. Acad. Sci. U.S.A. 90 5989–5993 (1993).
Gupta, P.K., Pandrangi, R.G., Lee, M. & King, C.M. Induction of mutations by N-acetoxy-N-acetyl-2-aminofluorene modified M13 viral DNA. Carcinogenesis 12, 819–824 (1991).
O'Handley, S.F. et al. Structural characterization of an N-acetyl-2-aminofluorene (AAF) modified DNA oligomer by NMR, energy minimization, and molecular dynamics. Biochemistry 32, 2481–2481 (1993).
Evans, F.E., Miller, D.W. & Levine, R.A. 1H NMR Study of self-association and restricted internal rotation of the C8-substituted deoxyguanosine 5′-monophosphate adduct of the carcinogen 2-(acetylamino)fluorene. J. Biomolec. Struct. Dyn. 3, 935–948 (1986).
Cho, B.P., Beland, F.A. & Marques, M.M. NMR stuctural studies of a 15-mer DNA duplex from a ras protooncogene modified with the carcinogen 2-Amniofluorene: conformational heterogeneity. Biochemistry (1994) in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eckel, L., Krugh, T. 2–Aminofluorene modified DNA duplex exists in two interchangeable conformations. Nat Struct Mol Biol 1, 89–94 (1994). https://doi.org/10.1038/nsb0294-89
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0294-89
This article is cited by
-
Mechanism of error-free and semitargeted mutagenic bypass of an aromatic amine lesion by Y-family polymerase Dpo4
Nature Structural & Molecular Biology (2010)