Abstract
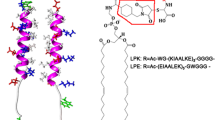
In membrane proteins, the extent to which polarity, hydrogen bonding, and van der Waals packing interactions of the buried, internal residues direct protein folding and association of transmembrane segments is poorly understood. The energetics associated with these various interactions should differ substantially between membrane versus water-soluble proteins. To help evaluate these energetics, we have altered a water-soluble, two-stranded coiled-coil peptide to render its sequence soluble in membranes. The membrane-soluble peptide associates in a monomer-dimer-trimer equilibrium, in which the trimer predominates at the highest peptide/detergent ratios. The oligomers are stabilized by a buried Asn side chain. Mutation of this Asn to Val essentially eliminates oligomerization of the membrane-soluble peptide. Thus, within a membrane-like environment, interactions involving a polar Asn side chain provide a strong thermodynamic driving force for membrane helix association.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
O'Shea, E.K., Klemm, J.D., Kim, P.S. & Alber, T.A. X-ray structure of the GCN4 leucine zipper, a two-stranded coiled coil. Science 254, 539–544 ( 1991).
Harbury, P.B., Zhang, T., Kim, P.S. & Alber, T. A switch between two-, three-, and four-stranded coiled coils. Science 262, 1401–1407 (1993).
Harbury, P.B., Kim, P.S. & Alber, T. Crystal structure of an isoleucine-zipper trimer. Nature 371, 80–83 ( 1994).
Lemmon, M.A. et al. Glycophorin A Dimerization is Driven by Specific Interactions between Transmembrane alpha Helices. J. Biol. Chem. 267, 7683–7689 (1991).
Arkin, I.T. et al. Structural organization of the pentameric transmembrane alpha-helices of phospholamban, a cardiac ion channel. EMBO J. 13 , 4757–4764 (1994).
Simmerman, H.K., Kobayashi, Y.M., Autry, J.M. & Jones, L.R. A leucine zipper stabilizes the pentameric membrane domain of phospholamban and forms a coiled-coil pore structure. J. Biol. Chem. 271, 5941–5946 (1996).
Reddy, L.G., Jones, L.R. & Thomas, D.D. Depolymerization of phospholamban in the presence of calcium pump: a fluorescence energy transfer study. Biochemistry 38, 3954–3962 ( 1999).
London, E. & Khorana, H.G. Denaturation and renaturation of bacteriorhodopson. J. Biol. Chem. 257, 7003–7011 (1982).
Chung, L.A., Lear, J.D. & DeGrado, W.F. Fluorescence studies of the secondary structure and orientation of a model ion channel peptide in phospholipid vesicles. Biochemistry 31, 6608–6616 (1992).
Li, M. et al. A fluorescence energy transfer method for analyzing protein oligomeric structure: application to phospholamban Biophys. J. 76, 2587–2599 (1999).
Adair, B.D. & Engelman, D.M. Glycophorin A helical transmembrane domains dimerize in phospholipid bilayers: A resonance energy transfer study . Biochemistry 33, 5539– 5544 (1994).
Vorherr, T., Wrzosek, A., Chiesi, M. & Carafoli, E. Total synthesis and functional properties of the membrane-intrinsic protein phospholamban . Protein Sci. 2, 339–347 (1993).
Tanford, C., Nozaki, Y., Reynolds, J.A. & Makino, S. Molecular characterization of proteins in detergent solutions. Biochemistry 13, 2369–2376 (1974).
Gonzalez,L., Brown, R.A., Richardson, D. & Alber, T. Crystal structures of a single coiled-coil peptide in two olomeric states reveal the basis for structural polymorphism. Nature Struct. Biol. 3, 1002–1010 ( 1996).
Zhou, F.X., Cocco, M.J., Russ, W.P., Brunger, A.T. & Engleman, D.M. Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nature Struct. Biol. 7, 154–160 (2000).
Weis, W.I., Brunger, A.T., Skehel, J.J. & Wiley, D.C. Refinement of the influenza virus hemagglutinin by simulated annealing. J. Mol. Biol. 212, 737–761 (1990).
Schneider, J.P., Lombardi, A. & DeGrado, W.F. Analysis and design of three-stranded coiled coils and three-helix bundles. Folding & Design 3, R29–40 (1998).
Stevens, T.J. & Arkin, I.T. Are membrane proteins “inside-out” proteins. Proteins 36, 135– 143 (1999).
Kochendoerfer, G.G., Salom, D., Lear, J., Kent, S. & DeGrado, W.F. Total chemical synthesis of the integral membrane protein influenza A virus M2: role of its C-terminal domain in tetramer assembly. Biochemistry 38, 11905–11913 (1999).
Fleming, K.G., Ackerman, A.L. & Engelman, D.M. The effect of point mutations on the free energy of transmembrane alpha- helix dimerization. J. Mol. Biol. 272, 266–275 (1997).
Laue, T., Shaw, B.D., Ridgeway, T.M. & Pelletier, S.L. in Analytical ultracentrifugation in biochemistry and polymer science (eds Harding, S.E., Rowe, A.J. & Horton, J.C.) 90– 125 (The Royal Society of Chemistry, Cambridge, UK; 1992).
Kharakoz, D.P. Partial volumes and compressibilities of extended polypeptide chains in aqueous solution: addititivity scheme and implication of protein unfolding at normal and high pressure. Biochemistry. 36, 10276 –10285 (1997).
Popot, J.L., Gerchman, S.E. & Engelman, D.M. Refolding of bacteriorhodopsin in lipid bilayers. A thermodynamically controlled two-stage process. J. Mol. Biol. 198, 655–676 ( 1987).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Choma, C., Gratkowski, H., Lear, J. et al. Asparagine-mediated self-association of a model transmembrane helix. Nat Struct Mol Biol 7, 161–166 (2000). https://doi.org/10.1038/72440
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72440
This article is cited by
-
Computational Simulation of Holin S105 in Membrane Bilayer and Its Dimerization Through a Helix-Turn-Helix Motif
The Journal of Membrane Biology (2021)
-
Small-residue packing motifs modulate the structure and function of a minimal de novo membrane protein
Scientific Reports (2020)
-
An intramembrane chaperone complex facilitates membrane protein biogenesis
Nature (2020)
-
The peptide hormone glucagon forms amyloid fibrils with two coexisting β-strand conformations
Nature Structural & Molecular Biology (2019)
-
A comprehensive computational study of amino acid interactions in membrane proteins
Scientific Reports (2019)