Abstract
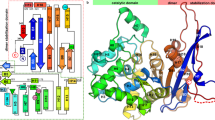
The crystal structure of E. coli asparagine synthetase has been determined by X-ray diffraction analysis at 2.5 Å resolution. The overall structure of the enzyme is remarkably similar to that of the catalytic domain of yeast aspartyl-tRNA synthetase despite low sequence similarity. These enzymes have a common reaction mechanism that implies the formation of an aminoacyl-adenylate intermediate. The active site architecture and most of the catalytic residues are also conserved in both enzymes. These proteins have probably evolved from a common ancestor even though their sequence similarities are small. The functional and structural similarities of both enzymes suggest that new enzymatic activites would generally follow the recruitment of a protein catalyzing a similiar chemical reaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schimmel, P., Annu. Rev. Biochem. 56, 125–158 (1987).
Meister, A. in The Enzymes (ed. Boyer, P.D.) 561–580 (Academic Press, New York; 1974).
Cusack, S. Nature Struct. Biol. 2, 824–831 (1995).
Ruff, M., et al. Science 252, 1682–1689 (1991).
Gatti, D.L. & Tzagoloff, A. J. Mol. Biol. 218, 557–568 (1991).
Hinchman, S.K., Henikoff, S. & Schuster, S.M.J. Biol. Chem. 267, 144–149 (1992).
Cedar, H. & Schwartz, J.H. J. Biol. Chem. 244, 4112–4121 (1969).
Cedar, H. & Schwartz, J.H. J. Biol. Chem. 244, 4122–4127 (1969).
Yamaguchi, H., et al. J. Mol. Biol. 299, 1083–1100 (1993).
Almassy, R.J., Janson, C.A., Hamlin, R., Xuong, N.-H. & Eisenberg, D. Nature 325, 304–309 (1986).
Arnez, J.G. et al. EMBO J. 14, 4143–4155 (1995).
Eriani, G. et al. Proc. Natl. Acad. Sci. 90, 10816–10820 (1993).
Eriani, G., Deralue, M., Poch, O., Gangloff, J. & Moras, D. Nature 347, 203–206 (1990).
Cavarelli, J., et al. EMBO J. 13, 327–337 (1994).
Poterszman, A., Delarue, M., Thierry, J.-C. & Moras, D. J. Mol. Biol. 244, 158–167 (1994).
Deralue, M., et al. EMBO J. 13, 3219–3229 (1994).
Martin, F., et al. J. Mol. Biol. 234, 965–974 (1993).
Imanaka, T., Lee, S., Takagi, M. & Fujiwara, S. Gene 164, –1995 (1995).
Neidhart, D.J., Fenyon, G.L., Gerlt, J.A. & Petsko, G.A. Nature 347, 692–694 (1990).
Nakatsu, T., Kato, H. & Oda, J. Acta Crystallogr. D 52, 604–606 (1996).
Matthews, B.W. & Rossman, M.G. Meth. Enzymol. 115, 397–420 (1985).
Sato, M., et al. J. Appl. Crystallogar. 25, 348–357 (1992).
Furey, W. & Swaminathan, S., Meth. Enzymol. 277, 590–620 (1997).
Wang, B.-C. Meth. Enzymol. 115, 90–112 (1985).
Bricogne, G. Acta Crystallogr. A 32, 832–847 (1976).
Jones, T.A. J. Appl. Crystallogr. 11, 268–272 (1978).
Br¨nger, A.T., Kuriyan, J. & Karplus, M. Science 235, 458–460 (1987).
Laskowski, R.A. J. Appl. Crystallogr. 26, 283–291 (1993).
Klraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakatsu, T., Kato, H. & Oda, J. Crystal structure of asparagine synthetase reveals a close evolutionary relationship to class II aminoacyl-tRNA synthetase. Nat Struct Mol Biol 5, 15–19 (1998). https://doi.org/10.1038/nsb0198-15
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0198-15
This article is cited by
-
Saccharomyces cerevisiae ASN1 and ASN2 are asparagine synthetase paralogs that have diverged in their ability to polymerize in response to nutrient stress
Scientific Reports (2019)
-
Targeting adenylate-forming enzymes with designed sulfonyladenosine inhibitors
The Journal of Antibiotics (2019)
-
Unusual domain architecture of aminoacyl tRNA synthetases and their paralogs from Leishmania major
BMC Genomics (2012)
-
Systematic search for putative new domain families in Mycoplasma gallisepticum genome
BMC Research Notes (2010)