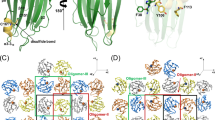
Abstract
Shigella dysenteriae is the pathogen responsible for the severe form of dysentery in humans. It produces Shiga toxin, the prototype of a family of closely related bacterial protein toxins. We have determined the structure of the holotoxin, an AB5 hexamer, by X–ray crystallography. The five B subunits form a pentameric ring, encircling a helix at the carboxy terminus of the A subunit. The A subunit interacts with the B pentamer via this C–terminal helix and a four–stranded mixed β–sheet. The fold of the rest of the A subunit is similar to that of the A chain of the plant toxin ricin; both are N–glycosidases. However, the active site in the bacterial holotoxin is blocked by a segment of polypeptide chain. These residues of the A subunit would be released as part of the activation mechanism of the toxin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
O'Brien, A.D. et al. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Topics Microbiol. Immunol. 180, 65–94 (1992).
Endo, Y. et al. Site of action of Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171, 45–50 (1988).
Olsnes, S., Reisbig, R. & Eiklid, K. Subunit structure of Shigella cytotoxin. J. biol. Chem. 256, 8732–8738 (1981).
Katzin, B.J., Collins, E.J. & Robertus, J.D. Structure of ricin A-chain at 2.5 Å. Proteins 10, 251–259 (1991).
Jacewicz, M., Clausen, H., Nudelman, E., Donohue-Rolfe, A. & Keusch, G.T. Pathogenesis of Shigella diarrhea. XI. Isolation of a Shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriosylceramide. J. exp. Med. 163, 1391–1404 (1986).
Cohen, A., Hannigan, G.E., Williams, B.R.G. & Lingwood, C.A. Roles of globotriosyl- and galabiosylceramide in verotoxin binding and high affinity interferon receptor. J. biol. Chem. 262, 17088–17091 (1987).
Connolly, M.L. Solvent-accessible surfaces of proteins and nucleic acids. Science 221, 709–713 (1983).
Monzingo, A.F. & Robertus, J.D. X-ray analysis of substrate analogs in the ricin A-chain active site. J. molec. Biol. 227, 1136–1145 (1992).
Reisbig, R., Olsnes, S. & Eiklid, K. The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60S ribosomal subunit. J. biol. Chem. 256, 8739–8744 (1981).
Sielecki, A.R. & James, M.N.G. Molecular structure of an aspartic proteinase zymogen, porcine pepsinogen, at 1.8 Å resolution. Nature 319, 33–38 (1986).
Stein, P.E., Boodhoo, A., Tyrrell, G.J., Brunton, J.L. & Read, R.J. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature 355, 748–750 (1992).
Sixma, T.K. et al. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature 351, 371–377 (1991).
Sixma, T.K. et al. Lactose binding to heat-labile enterotoxin revealed by X-ray crystallography. Nature 355, 561–564 (1992).
Kozlov, Y.V., Chernaia, M.M., Fraser, M.E. & James, M.N.G. Purification and crystallization of Shiga toxin from Shigella dysenteriae. J. molec. Biol. 232, 704–706 (1993).
Sakabe, N. X-ray diffraction data collection system for modern protein crystallography with a Weissenberg camera and an imaging plate using synchrotron radiation. Nucl. Instrum. Meth. A 303, 448–463 (1991).
Sixma, T.K., Stein, P.E., Hol, W.G.J. & Read, R.J. Comparison of the B-pentamers of heat-labile enterotoxin and verotoxin-1: two structures with remarkable similarity and dissimilarity. Biochemistry 32, 191–198 (1993).
Navaza, J. On the fast rotation function. Acta Crystallogr. A43, 645–653 (1987).
Navaza, J. Accurate computation of the rotation matrices. Acta Crystallogr. A46, 619–620 (1990).
Fujinaga, M. & Read, R.J. Experiences with a new translation-function program. J. appl. Crystallogr. 20, 517–521 (1987).
Otwinowski, Z. Accurate refinement of heavy atom parameters. Amer. Crystallogr. Assoc. 1990 Annual Meeting, New Orleans, Abstr. C04 (1990).
Jones, T.A. Interactive computer graphics: FRODO. With modifications for TOM UQV/CIT v.2.8.0 by C. Cambillau, M. Israel, G. Griffin and A. Chirino. Meth. Enzym. 115, 157–171 (1985).
Read, R.J. Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A42, 140–149 (1986).
Zuker, M. Suboptimal sequence alignment in molecular biology. Alignment with error analysis. J. molec. Biol. 221, 403–420 (1991).
Quanta Release 3.2, Polygen Corporation, Waltham, MA, USA (1991).
Brünger, A.T. X-PLOR Manual, Version 2.1, Yale University, New Haven, CT, USA (1990).
Vellieux, F.M.D. et al. Structure of glycosomal glyceraldehyde-3-phosphatedehydrogenasefrom Trypanosoma barucei determined from Laue data. Proc. natn. Acad. Sci. U.S.A. 90, 2355–2359 (1993).
Tronrud, D.E. Conjugate-direction minimization. An improved method for the refinement of macromolecules. Acta Crystallogr. A48, 912–916 (1992).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. appl. Crystallogr. 24, 946–950 (1991).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure:pattern recognition of hydrogen bonded and geometrical features. Biopolymers 22, 2577–2637 (1983).
Jones, T.A. O. Version 5.8.1, Uppsala, Sweden (1992).
SUPPOS is from the BIOMOL package, Gröningen, Holland.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fraser, M., Chernaia, M., Kozlov, Y. et al. Crystal structure of the holotoxino from Shigella dysenteriae at 2.5 Å resolution. Nat Struct Mol Biol 1, 59–64 (1994). https://doi.org/10.1038/nsb0194-59
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0194-59
This article is cited by
-
The Fatal Role of Enterohaemorrhagic Escherichia coli Shiga Toxin-associated Extracellular Vesicles in Host Cells
Journal of Microbiology (2023)
-
Functional dissection of the retrograde Shiga toxin trafficking inhibitor Retro-2
Nature Chemical Biology (2020)
-
Shiga toxin signals via ATP and its effect is blocked by purinergic receptor antagonism
Scientific Reports (2019)
-
Degradation and inactivation of Shiga toxins by nitrogen gas plasma
AMB Express (2017)
-
Development of camelid single chain antibodies against Shiga toxin type 2 (Stx2) with therapeutic potential against Hemolytic Uremic Syndrome (HUS)
Scientific Reports (2016)