Abstract
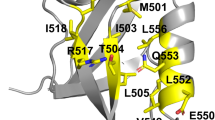
We used a novel charge optimization technique to study the small ribonuclease barnase and to analyze its interaction with a natural tight binding inhibitor, the protein barstar. The approach uses a continuum model to explicitly determine the charge distributions that lead to the most favorable electrostatic contribution to binding when competing desolvation and interaction effects are included. Given its backbone fold, barstar is electrostatically optimized for tight binding to barnase when compared with mutants where residues have been substituted with one of the 20 common amino acids. Natural proteins thus appear to use optimization of electrostatic interactions as one strategy for achieving tight binding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Janin, J. & Chothia, C. J. Biol. Chem. 265, 16027–16030 (1990).
Hendsch, Z.S. & Tidor, B. Protein Sci. 3, 211–226 (1994).
Yang, A.-S. & Honig, B. J. Mol. Biol. 252, 351–365 (1995).
Wang, L., O'Connell, T., Tropsha, A. & Hermans, J. Biopolymers 39, 479–489 (1996).
Waldburger, C.D., Schildbach, J.F. & Sauer, R.T. Nature Struct. Biol. 2, 122–128 (1995)
Wimley, W.C., Gawrisch, K., Creamer, T.P. & White, S.H. Proc. Natl. Acad. Sci. USA 93, 2985– 2990 (1996).
Sindelar, C.V., Hendsch, Z.S. & Tidor, B. Protein Sci. 7, 1898–1914 (1998).
O'Shea, E.K., Rutkowski, R. & Kim, P.S. Cell 68, 699– 708 (1992).
Lee, L.-P. & Tidor, B. J. Chem. Phys. 106, 8681–8690 (1997).
Kangas, E. & Tidor, B. J. Chem. Phys. 109, 7522–7545 (1998).
Schreiber, G. & Fersht, A.R. Biochemistry 32, 5145–5150 (1993).
Hartley, R.W. Biochemistry 32, 5978–5984 (1993).
Buckle, A.M., Schreiber, G. & Fersht, A.R. Biochemistry 33, 8878– 8889 (1994).
Ratnaparkhi, G.S. Ramachandran, S., Udgaonkar, J.B. & Varadarajan, R. Biochemistry 37, 6958– 6966 (1998).
Schreiber, G. & Fersht, A.R. J. Mol. Biol. 248, 478–486 (1995).
Schreiber, G., Buckle, A.M & Fersht, A.R. Structure 2, 945 –951 (1994).
Schreiber, G. & Fersht, A.R. Nature Struct. Biol. 3, 427–431 ( 1996).
Gabdoulline, R.R. & Wade, R.C. Biophys. J. 72, 1917–1929 (1997).
Selzer, T. & Schreiber, G. J. Mol. Biol. 287, 409–419 (1999).
Hendsch, Z.S. & Tidor, B. Protein Sci. 8, 1381–1392 (1999).
Clackson, T. & Wells, J.A. Science 267, 383–386 (1995).
Brooks, B.R. et al. J. Comput. Chem. 4, 187– 217 (1983).
Dunbrack, R.L., Jr. & Karplus, M. J. Mol. Biol. 230, 543–574 (1993).
Gilson, M.K., Sharp, K.A. & Honig, B.H. J. Comput. Chem. 9, 327– 335 (1988).
Vanderbei, R.J. LOQO v4.01 (Princeton University, Princeton, New Jersey; 1998).
Kraulis, P.J. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt, E.A. & Bacon, D.J. Methods Enzymol. 277, 505–524 (1997).
Acknowledgements
We thank B. Honig for making the delphi and grasp computer programs available, M. Karplus for charmm, R.J. Vanderbei for loqo, and members of our research group, particularly J.A. Caravella and M.A. Ohliger, for helpful discussions and critical reading of an earlier draft of the manuscript. This work was supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, LP., Tidor, B. Barstar is electrostatically optimized for tight binding to barnase. Nat Struct Mol Biol 8, 73–76 (2001). https://doi.org/10.1038/83082
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83082
This article is cited by
-
Recombinant expression of Barnase in Escherichia coli and its application in plasmid purification
Microbial Cell Factories (2021)
-
Trypsin-Ligand binding affinities calculated using an effective interaction entropy method under polarized force field
Scientific Reports (2017)
-
Interaction specific binding hotspots in Endonuclease colicin-immunity protein complex from MD simulations
Science China Chemistry (2013)
-
Designing electrostatic interactions in biological systems via charge optimization or combinatorial approaches: insights and challenges with a continuum electrostatic framework
Theoretical Chemistry Accounts (2012)
-
On the electrostatic component of protein-protein binding free energy
PMC Biophysics (2008)