Abstract
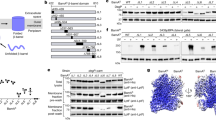
The (βα)8-barrel, which is the most frequently encountered protein fold, is generally considered to consist of a single structural domain. However, the X-ray structure of the imidazoleglycerol phosphate synthase (HisF) from Thermotoga maritima has identified it as a (βα)8-barrel made up of two superimposable subdomains (HisF-N and HisF-C). HisF-N consists of the four N-terminal (βα) units and HisF-C of the four C-terminal (βα) units. It has been postulated, therefore, that HisF evolved by tandem duplication and fusion from an ancestral half-barrel. To test this hypothesis, HisF-N and HisF-C were produced in Escherichia coli, purified and characterized. Separately, HisF-N and HisF-C are folded proteins, but are catalytically inactive. Upon co-expression in vivo or joint refolding in vitro, HisF-N and HisF-C assemble to the stoichiometric and catalytically fully active HisF-NC complex. These findings support the hypothesis that the (βα)8-barrel of HisF evolved from an ancestral half-barrel and have implications for the folding mechanism of the members of this large protein family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pujadas, G. & Palau, J. Biologia Bratislava 54, 231–254 (1999).
Thoma, R., Hennig, M., Sterner, R. & Kirschner, K. Structure Fold. Des. 8, 265–276 ( 2000).
Higgins, W., Fairwell, T. & Miles, E.W. Biochemistry 18, 4827– 4835 (1979).
Eder, J. & Kirschner, K. Biochemistry 31, 3617–3625 (1992).
Bertolaet, B.L. & Knowles, J.R. Biochemistry 34, 5736–5743 ( 1995).
Klem, T.J. & Davisson, V.J. Biochemistry 32, 5177–5186 (1993).
Lang, D.A., Thoma, R., Henn-Sax, M., Sterner, R. & Wilmanns, M. Science 289, 1546 –1550 (2000).
Thoma, R., Schwander, M., Liebl, W., Kirschner, K. & Sterner, R. A. Extremophiles 2, 379–389 (1998).
Studier, F.W. & Moffatt, B.A. J. Mol. Biol. 189, 113–130 (1986).
Johnson, W.C. Jr. Proteins 7, 205– 214 (1990).
Greenfield, N.J. Anal. Biochem. 235, 1–10 (1996).
Schmid, F.X. In Protein structure: a practical approach, 2nd Edition (ed. Creighton, T.E.), 259–295 (IRL Press, Oxford; 1997).
Hubbard, S.J. Biochim. Biophys. Acta 1382, 191–206 (1998).
Beismann-Driemeyer, S. Ph.D. thesis, Universität Köln, Germany ( 2000).
Thoma, R. et al. FEBS Lett. 454, 1–6 (1999).
Knowles, J.R. Nature 350, 121–124 ( 1991).
Jaenicke, R. Prog. Biophys. Mol. Biol. 71, 155– 241 (1999).
Terwilliger, T.C. Adv. Protein. Chem. 46, 177–215 (1995).
Erickson, H.P. J. Mol. Biol. 206, 465–474 (1989).
Nagi, A.D. & Regan, L. Fold. Des. 2, 67–75 (1997).
Marcotte, E.M. et al. Science 285, 751–753 (1999).
Heinz, D.W., Essen, L.-O. & Williams, R.L. J. Mol. Biol. 275, 635– 650 (1998).
Waddle, J.J., Johnston, T.C. & Baldwin, T.O. Biochemistry 26, 4917– 4921 (1987).
Zitzewitz, J.A., Gualfetti, P.J., Perkons, I.A., Wasta, S.A. & Matthews, C.R. Protein Sci. 8,1200–1209 ( 1999).
Wilmanns, M., Hyde, C.C., Davies, D.R., Kirschner, K. & Jansonius, J.N. Biochemistry 30, 9161–9169 (1991).
Sarkar, G. & Sommer, S.S. BioTechniques 8, 404–407 (1990).
Laemmli, U.K. Nature 227, 680–685 ( 1970).
Bradford, M.M. Anal. Biochem. 72, 248–254 (1976).
Pace, C.N., Vajdos, F., Fee, L., Grimsley, G. & Gray T. Protein Sci. 4, 2411 –2423 (1995).
Schägger, H. & von Jagow, G. Anal. Biochem. 166, 368–379 ( 1987).
Hommel, U., Eberhard, M. & Kirschner, K. Biochemistry 34, 5429– 5439 (1995).
Acknowledgements
We thank H.-J. Fritz, K. Kirschner, D. Lang, R. Thoma and M. Wilmanns for support and helpful discussions. This work was sponsored by grants from the Deutsche Forschungsgemeinschaft and a Heisenberg-fellowship to R.S. This paper is dedicated to Professor Marianne Baudler on the occasion of her 80th birthday.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Höcker, B., Beismann-Driemeyer, S., Hettwer, S. et al. Dissection of a (βα)8-barrel enzyme into two folded halves. Nat Struct Mol Biol 8, 32–36 (2001). https://doi.org/10.1038/83021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83021
This article is cited by
-
The Fate of Duplicated Enzymes in Prokaryotes: The Case of Isomerases
Journal of Molecular Evolution (2023)
-
Heme-binding enables allosteric modulation in an ancient TIM-barrel glycosidase
Nature Communications (2021)
-
Simultaneously Improved Thermostability and Hydrolytic Pattern of Alpha-Amylase by Engineering Central Beta Strands of TIM Barrel
Applied Biochemistry and Biotechnology (2020)
-
Expression patterns of alpha-amylase and beta-amylase genes provide insights into the molecular mechanisms underlying the responses of tea plants (Camellia sinensis) to stress and postharvest processing treatments
Planta (2019)
-
Highly active enzymes by automated combinatorial backbone assembly and sequence design
Nature Communications (2018)