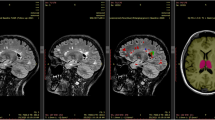
Abstract
Over the past 15 years, MRI lesion activity has become the accepted surrogate primary outcome measure in proof-of-concept placebo-controlled clinical trials of new immunomodulating therapies in relapse-onset multiple sclerosis (MS). In parallel, the number of patients that are available for the placebo arm of trials has declined, and more-aggressive drugs are being developed. A critical review is warranted to ensure efficient MRI—and patient—resource utilization. Recently, an international panel reviewed the methodology for efficient use of MRI-monitored trials in relapse-onset MS. In this article, we provide up-to-date recommendations for scan acquisition, image analysis, outcome-measure definition and standards of reporting. Factors to consider for optimizing trial design, such as outcome measure selection and the unique requirements of phase II and phase III trials, including active-comparator studies, are outlined. Finally, we address safety considerations in the use of MRI in MS trials, and the safety-related responsibilities of the various parties involved in conducting such trials.
Key Points
-
Active lesions on MRI scans are accepted as a surrogate for disease activity in relapsing–remitting multiple sclerosis and as the primary outcome in proof-of-concept phase II studies of immunomodulation
-
New post-processing techniques will increase the sensitivity and accuracy of MRI to detect active lesions, and will reduce the amount of contrast material needed
-
In definitive phase III trials, clinical end points remain primary, but MRI scans provide important information about subgroup performance
-
In clinical trials evaluating new therapies with unknown or more-aggressive mechanisms of action, MRI conveys important safety information
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Miller, D. H. et al. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J. Neurol. Neurosurg. Psychiatry 54, 683–688 (1991).
Miller, D. H. et al. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. US National MS Society Task Force. Ann. Neurol. 39, 6–16 (1996).
Miller, D. H. et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003).
O'Connor, P. W. et al. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66, 894–900 (2006).
Kappos, L. et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N. Engl. J. Med. 355, 1124–1140 (2006).
Kappos, L. et al. Efficacy and safety of oral fumarate in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372, 1463–1472 (2008).
Wynn, D. et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol. 9, 381–390 (2010).
Polman, C. H. et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology 64, 987–991 (2005).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
van Oosten, B. W. et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 47, 1531–1534 (1996).
[No authors listed] TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 53, 457–465 (1999).
Barkhof, F., van Waesberghe, J. H., Uitdehaag, B. M. & Polman, C. H. Ibuprofen does not suppress active multiple sclerosis lesions on gadolinium-enhanced MR images. Ann. Neurol. 42, 982 (1997).
Polman, C. H. et al. Oral interferon beta-1a in relapsing–remitting multiple sclerosis: a double-blind randomized study. Mult. Scler. 9, 342–348 (2003).
Kappos, L., Barkhof, F. & Desmet, A. The effect of oral temsirolimus on new magnetic resonance imaging scan lesions, brain atrophy, and the number of relapses in multiple sclerosis: results from a randomised, controlled trial. J. Neurol. 252 (Suppl. 2), 46 (2005).
Segal, B. M. et al. Ustekinumab MS Investigators. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing–remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7, 796–804 (2008).
Filippi, M., Wolinsky, J. S., Comi, G. & CORAL Study Group. Effects of oral glatiramer acetate on clinical and MRI-monitored disease activity in patients with relapsing multiple sclerosis: a multicentre, double-blind, randomised, placebo-controlled study. Lancet Neurol. 5, 213–220 (2006).
Fazekas, F. et al. MRI results from the European Study on Intravenous Immunoglobulin in Secondary Progressive Multiple Sclerosis (ESIMS). Mult. Scler. 11, 433–440 (2005).
Barkhof, F. et al. Ibudilast in relapsing–remitting multiple sclerosis: a neuroprotectant? Neurology 74, 1033–1040 (2010).
Killestein, J. et al. Antibody-mediated suppression of Vβ5.2/5.3+ T cells in multiple sclerosis: results from an MRI-monitored phase II clinical trial. Ann. Neurol. 51, 467–474 (2002).
Polman, C. H. et al. Ethics of placebo-controlled clinical trials in multiple sclerosis: a reassessment. Neurology 70, 1134–1140 (2008).
O'Connor, P. et al. 250 μg or 500 μg interferon beta-1b versus 20 mg glatiramer acetate in relapsing–remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 8, 889–897 (2009).
Mikol, D. D. et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 7, 903–914 (2008).
Inusah, S. et al. Assessing changes in relapse rates in multiple sclerosis. Mult. Scler. 16, 1414–1421 (2010).
Barkhof, F. & Filippi, M. MRI—the perfect surrogate marker for multiple sclerosis? Nat. Rev. Neurol. 5, 182–183 (2009).
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8, 431–440 (1989).
Sormani, M. P. et al. MRI metrics as surrogate markers for clinical relapse rate in relapsing–remitting MS patients. Neurology 58, 417–421 (2002).
Sormani, M. P. et al. MRI metrics as surrogate endpoints for EDSS progression in SPMS patients treated with IFN β-1b. Neurology 60, 1462–1466 (2003).
Sormani, M. P. et al. Magnetic resonance active lesions as individual-level surrogate for relapses in multiple sclerosis. Mult. Scler. 17, 541–549 (2011).
Petkau, J. et al. Magnetic resonance imaging as a surrogate outcome for multiple sclerosis relapses. Mult. Scler. 14, 770–778 (2008).
Daniels, M. & Hughes, M. D. Meta-analysis for the evaluation of potential surrogate markers. Stat. Med. 16, 1965–1982 (1997).
Korn, E. L., Albert, P. S. & McShane, L. M. Assessing surrogates as trial endpoints using mixed models. Stat. Med. 24, 163–182 (2005).
Sormani, M. P. et al. Magnetic resonance imaging as a potential surrogate for relapses in MS: a meta-analytic approach. Ann. Neurol. 65, 268–275 (2009).
Sormani, M., Bonzano, L., Roccatagliata, L. & de Stefano, N. Magnetic resonance imaging as surrogate for clinical endpoints in multiple sclerosis: data on novel oral drugs. Mult. Scler. 17, 630–633 (2011).
Sormani, M. P. et al. Surrogate endpoints for EDSS worsening in multiple sclerosis. A meta-analytic approach. Neurology 75, 302–309 (2010).
Wattjes, M. P. & Barkhof, F. High field MRI in the diagnosis of multiple sclerosis: high field—high yield? Neuroradiology 51, 279–292 (2009).
Geurts, J. J. et al. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236, 254–260 (2005).
Calabrese, M. et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch. Neurol. 64, 416–422 (2007).
Nelson, F. et al. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am. J. Neuroradiol. 28, 1645–1649 (2007).
Moraal, B. et al. Multi-contrast, isotropic, single-slab 3D MR imaging in multiple sclerosis. Eur. Radiol. 18, 2311–2320 (2008).
Barkhof, F., Pouwels, P. J. & Wattjes, M. P. The Holy Grail in diagnostic neuroradiology: 3T or 3D? Eur. Radiol. 21, 449–456 (2011).
Bot, J. C. & Barkhof, F. Spinal-cord MRI in multiple sclerosis: conventional and nonconventional MR techniques. Neuroimaging Clin. N. Am. 19, 81–99 (2009).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011).
Thorpe, J. W. et al. Serial gadolinium-enhanced MRI of the brain and spinal cord in early relapsing–remitting multiple sclerosis. Neurology 46, 373–378 (1996).
Silver, N. C. et al. A modified protocol to improve the detection of enhancing brain and spinal cord lesions in multiple sclerosis. J. Neurol. 248, 215–224 (2001).
Losseff, N. A. et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 119, 701–708 (1996).
Kalkers, N. F., Barkhof, F., Bergers, E., van Schijndel, R. & Polman, C. H. The effect of the neuroprotective agent riluzole on MRI parameters in primary progressive multiple sclerosis: a pilot study. Mult. Scler. 8, 532–533 (2002).
Silver, N. C. et al. Sensitivity of contrast enhanced MRI in multiple sclerosis. Effects of gadolinium dose, magnetization transfer contrast and delayed imaging. Brain 120, 1149–1161 (1997).
Comi, G. et al. Effect of laquinimod on MRI-monitored disease activity in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 371, 2085–2092 (2008).
Dousset, V. et al. MR imaging of relapsing multiple sclerosis patients using ultra-small-particle iron oxide and compared with gadolinium. AJNR Am. J. Neuroradiol. 27, 1000–1005 (2006).
Vellinga, M. M. et al. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain 131, 800–807 (2008).
Barkhof, F. et al. Improving interobserver variation in reporting gadolinium-enhanced MRI lesions in multiple sclerosis. Neurology. 49, 1682–1688 (1997).
Molyneux, P. D. et al. Visual analysis of serial T2-weighted MRI in multiple sclerosis: intra- and interobserver reproducibility. Neuroradiology 41, 882–888 (1999).
Rovaris, M. et al. Multiple sclerosis: interobserver agreement in reporting active lesions on serial brain MRI using conventional spin echo, fast spin echo, fast fluid-attenuated inversion recovery and post-contrast T1-weighted images. J. Neurol. 246, 920–925 (1999).
Filippi, M. et al. Effect of training and different measurement strategies on the reproducibility of brain MRI lesion load measurements in multiple sclerosis. Neurology 50, 238–244 (1998).
Sled, J. G., Zijdenbos, A. P. & Evans, A. C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 17, 87–97 (1998).
Cocosco, C. A., Zijdenbos, A. P. & Evans, A. C. A fully automatic and robust brain MRI tissue classification method. Med. Image Anal. 7, 513–527 (2003).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl. 1), S208–S219 (2004).
Horsfield, M. A. et al. Incorporating domain knowledge into the fuzzy connectedness framework: application to brain lesion volume estimation in multiple sclerosis. IEEE Trans. Med. Imaging 26, 1670–17680 (2007).
Klein, A. et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 46, 786–802 (2009).
Moraal, B. et al. Improved detection of active multiple sclerosis lesions: 3D subtraction imaging. Radiology 255, 154–163 (2010).
Moraal, B. et al. Long-interval T2-weighted subtraction magnetic resonance imaging: a powerful new outcome measure in multiple sclerosis trials. Ann. Neurol. 67, 667–675 (2010).
Barkhof, F., Calabresi, P. A., Miller, D. H. & Reingold, S. C. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat. Rev. Neurol. 5, 256–266 (2009).
Zivadinov, R. et al. Mechanisms of action of disease-modifying agents and brain volume changes in multiple sclerosis. Neurology 71, 136–144 (2008).
Kapoor, R. et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 9, 681–688 (2010).
Miller, D. H. et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology 68, 1390–1401 (2007).
Zhao, Y., Traboulsee, A., Petkau, A. J. & Li, D. Regression of new gadolinium enhancing lesion activity in relapsing–remitting multiple sclerosis. Neurology 70, 1092–1097 (2008).
Sormani, M. P. et al. Modelling MRI enhancing lesion counts in multiple sclerosis using a negative binomial model: implications for clinical trials. J. Neurol. Sci. 163, 74–80 (1999).
Cohen, J. A. et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362, 402–415 (2010).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
Barkhof, F. et al. Predicting gadolinium enhancement status in MS patients eligible for randomized clinical trials. Neurology 65, 1447–1454 (2005).
Jacobs, L. D. et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N. Engl. J. Med. 343, 898–904 (2000).
Barkhof, F. et al. Magnetic resonance imaging effects of interferon beta-1b in the BENEFIT study: integrated 2-year results. Arch. Neurol. 64, 1292–1298 (2007).
Shellock, F. G. & Spinazzi, A. MRI safety update 2008: part 1, MRI contrast agents and nephrogenic systemic fibrosis. AJR Am. J. Roentgenol. 191, 1129–1139 (2008).
Leiner, T. & Kucharczyk, W. NSF prevention in clinical practice: summary of recommendations and guidelines in the United States, Canada, and Europe. J. Magn. Reson. Imaging 30, 1357–1363 (2009).
Yousry, T. A. et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 354, 924–933 (2006).
Riddell, C. A. et al. Evaluation of safety monitoring guidelines based on MRI lesion activity in multiple sclerosis. Neurology (in press).
Hawker, K. et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 66, 460–471 (2009).
Miller, D. H. et al. Effect of interferon-β1b on magnetic resonance imaging outcomes in secondary progressive multiple sclerosis: results of a European multicenter, randomized, double-blind, placebo-controlled trial. European Study Group on Interferon-β1b in secondary progressive multiple sclerosis. Ann. Neurol. 46, 850–859 (1999).
Barkhof, F. et al. T1-hypointense lesions in secondary progressive multiple sclerosis: effect of interferon beta-1b treatment. Brain 124, 1396–1402 (2001).
Hartung, H. P. & Aktas, O. Evolution of multiple sclerosis treatment: next generation therapies meet next generation efficacy criteria. Lancet Neurol. 10, 293–295 (2011).
Acknowledgements
This work is based on a workshop of the Magnetic Resonance Imaging Network in Multiple Sclerosis (MAGNIMS) working group, co-sponsored by MAGNIMS, the US National Multiple Sclerosis Society and the Multiple Sclerosis Society of Canada, and supported by an unrestricted educational grant by Bayer-Schering Pharma. We thank A. Thompson and J. Palace for their helpful input.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article. F. Barkhof, J. H. Simon, F. Fazekas, M. Rovaris, L. Kappos, N. de Stefano, C. H. Polman, M. P. Sormani, D. K. Li, D. H. Miller and M. Filippi made substantial contributions to discussion of the article content. F. Barkhof, J. H. Simon, F. Fazekas, M. Rovaris, N. de Stefano, J. Petkau, M. P. Sormani, D. H. Miller and M. Filippi wrote the article. F. Barkhof, J. H. Simon, F. Fazekas, M. Rovaris, N. de Stefano, J. Petkau, M. P. Sormani, D. K. Li, P. O'Connor, D. H. Miller and M. Filippi contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F. Barkhof serves on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, GE Healthcare, Lundbeck, Merck Serono, Novartis, Sanofi-Aventis and Synthon . He has received speaker honoraria from Novartis, Serono Symposia, and BioClinica. He serves as a consultant for Sanofi-Aventis, Roche, Novartis, Biogen Idec, Jansen Alzheimer Immunotherapy, and GE Healthcare.
J. H. Simon declares no competing interests.
F. Fazekas has served on scientific advisory boards for Biogen Idec, Merck Serono, Novartis, Teva Pharmaceutical Industries, and Sanofi-Aventis. He has received travel support from Bayer Schering Pharma and Merck Serono, and research support from Biogen Idec, Merck Serono, Sanofi-Aventis and Teva Pharmaceutical Industries.
M. Rovaris has received funding for travel from Biogen-Dompé and Teva Pharmaceutical Industries. He has received speaker honoraria from Bayer Schering Pharma, Biogen-Dompé, Sanofi-Aventis and Teva Pharmaceutical Industries.
L. Kappos has received research support through the University Hospital Basel from Acorda Therapeutics, Actelion Pharmaceuticals, Advancell, Allozyne, Barofold, Bayer HealthCare Pharmaceuticals, Bayer Schering Pharma, Bayhill, Biogen Idec, BioMarin, Boehringer Ingelheim, CSL Behring, Geneuro, Genmab, GlaxoSmithKline, Glenmark, Merck Serono, MediciNova, Novartis, Sanofi-Aventis, Santhera Pharmaceuticals, Shire, Roche, Teva Pharmaceutical Industries, UCB and Wyeth, and also from the Swiss MS Society, the Swiss National Research Foundation, European Union,as well as Gianni Rubato, and Roche and Novartis Foundations.
N. De Stefano serves on a scientific advisory board for Merck Serono. He has received funding for travel from Merck Serono and Teva Pharmaceutical Industries. He has received speaker honoraria from Bayer Schering Pharma, Biogen-Dompé, BioMS Medical, Merck Serono and Teva Pharmaceutical Industries.
C. H. Polman serves on scientific advisory boards for and has received funding for travel and speaker honoraria from Actelion Pharmaceuticals, Bayer Schering Pharma, Biogen Idec, GlaxoSmithKline, Merck Serono, Novartis, Roche, Teva Pharmaceutical Industries and UCB. He receives research support from Biogen Idec, Merck Serono, Novartis and UCB.
J. Petkau has served on scientific advisory boards for Bayer Schering Pharma, Bayhill Therapeutics, Eisai, Merck Serono, Opexa Therapeutics, Schering-Plough and Solstice Neurosciences. He has received funding for travel and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Merck Serono/Pfizer and Solstice Neurosciences. He serves as a consultant for Bayer Schering Pharma, Bayhill Therapeutics, BTG International, Opexa Therapeutics, PRA International and Solstice Neurosciences. He receives research support from Bayer Schering Pharma and Opexa Therapeutics.
E. W. Radue has received payments through his institution for membership of advisory boards of Biogen Idec and Novartis, honoraria for consultancy from Biogen Idec, Bayer Schering Pharma, Merck, and Novartis, and for lecturing from Bayer Schering Pharma, Biogen Idec, and Novartis.
M. P. Sormani has received speaker honoraria from Biogen-Dompé, Biogen Idec, Merck Serono and Teva Pharmaceutical Industries. She serves as a consultant for Actelion Pharmaceuticals, Biogen Idec, Eidetica and Merck Serono.
D. K. Li has served on scientific advisory boards for Nuron Biotech and Roche. He serves on the speakers' bureaus for the Consortium of MS Centers, Merck Serono and Teva Pharmaceutical Industries. He serves as a consultant for Genzyme Corporation; performs MRI (50% clinical effort). He is the Director of the University of British Columbia MS/MRI Research Group, which has been contracted to perform central analysis of MRI scans for therapeutic trials with Angiotech, Bayer Schering Pharma, Berlex-Schering, BioMS Medical, Centocor (Janssen), Daiichi Sankyo, Genzyme Corporation, Roche, Merck Serono, Novartis, Sanofi-Aventis, Schering-Plough, Teva Pharmaceutical Industries and Transition Therapeutics, and receives research support from the MS Society of Canada and the Canadian Institute of Health Research.
P. O'Connor has received consulting fees and/or research support for MS trials from Actelion, Bayer, Biogen Idec, BioMS, Cognosci, Daiichi Sankyo, EMD Serono, Genentech and Genmab.
X. Montalban serves on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis and Teva Pharmaceutical Industries. He has received funding for travel and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis and Teva Pharmaceutical Industries. He serves as a consultant to Almirall, Bayer Schering Pharma, Biogen Idec, Eli Lilly and Company, Merck Serono, Novartis, Sanofi-Aventis and Teva Pharmaceutical Industries. He has received research support for clinical trials from Genentech, Genzyme and Wyeth.
D. H. Miller serves on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, GlaxoSmithKline and Novartis. He has received funding for travel or speaker honoraria from Bayer Schering Pharma, Biogen Idec, the Cleveland Clinic, GlaxoSmithKline, Novartis, the National MS Society. He receives publishing royalties for McAlpine's Multiple Sclerosis, fourth edition (Churchill Livingstone, 2005). He serves as a consultant for Biogen Idec and GlaxoSmithKline, and receives research support through his institution from Biogen Idec, GlaxoSmithKline, Novartis and Schering-Plough.
M. Filippi serves on scientific advisory boards for Genmab and Teva Pharmaceutical Industries, and has received funding for travel from Bayer Schering Pharma, Biogen-Dompé, Genmab, Merck Serono, and Teva Pharmaceutical Industries. He serves as a consultant to Bayer Schering Pharma, Biogen-Dompé, Genmab, Merck Serono, Pepgen Corporation and Teva Pharmaceutical Industries. He serves on speakers' bureaus for Bayer Schering Pharma, Biogen-Dompé, Genmab, Merck Serono and Teva Pharmaceutical Industries. He receives research support from Bayer Schering Pharma, Biogen-Dompé, Genmab, Merck Serono and Teva Pharmaceutical Industries.
Rights and permissions
About this article
Cite this article
Barkhof, F., Simon, J., Fazekas, F. et al. MRI monitoring of immunomodulation in relapse-onset multiple sclerosis trials. Nat Rev Neurol 8, 13–21 (2012). https://doi.org/10.1038/nrneurol.2011.190
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2011.190
This article is cited by
-
Automated Registration and Color Labeling of Serial 3D Double Inversion Recovery MR Imaging for Detection of Lesion Progression in Multiple Sclerosis
Journal of Digital Imaging (2022)
-
Longitudinal multi-centre brain imaging studies: guidelines and practical tips for accurate and reproducible imaging endpoints and data sharing
Trials (2019)
-
Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting
Nature Reviews Neurology (2018)
-
Future Brain and Spinal Cord Volumetric Imaging in the Clinic for Monitoring Treatment Response in MS
Current Treatment Options in Neurology (2018)
-
Disrupted topological organization of structural and functional brain connectomes in clinically isolated syndrome and multiple sclerosis
Scientific Reports (2016)