Key Points
-
Type 1 diabetes mellitus (T1DM) is an autoimmune disease characterized by the destruction of pancreatic β cells
-
An accumulating body of evidence suggests that T1DM is associated with loss of tolerance by autoreactive B cells
-
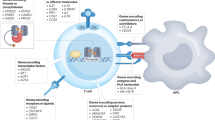
Although islet antigen-reactive B cells give rise to autoantibody secreting cells, their most important contribution to pathology in T1DM seems to be presentation of self-antigens to T cells
-
Loss of tolerance of islet-reactive B cells is associated with certain genetic polymorphisms
-
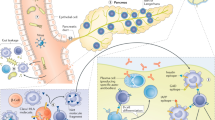
B cells contribute to diabetic kidney disease (DKD) through the production of antibodies that lead to the formation and deposition of immune complexes in the kidney
-
In a clinical trial, B cell-depletion therapy showed some efficacy in patients with T1DM; the development of non-cell depleting therapies might benefit patients with T1DM and DKD
Abstract
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder that affects an estimated 30 million people worldwide. It is characterized by the destruction of pancreatic β cells by the immune system, which leads to lifelong dependency on exogenous insulin and imposes an enormous burden on patients and health-care resources. T1DM is also associated with an increased risk of comorbidities, such as cardiovascular disease, retinopathy, and diabetic kidney disease (DKD), further contributing to the burden of this disease. Although T cells are largely considered to be responsible for β-cell destruction in T1DM, increasing evidence points towards a role for B cells in disease pathogenesis. B cell-depletion, for example, delays disease progression in patients with newly diagnosed T1DM. Loss of tolerance of islet antigen-reactive B cells occurs early in disease and numbers of pancreatic CD20+ B cells correlate with β-cell loss. Although the importance of B cells in T1DM is increasingly apparent, exactly how these cells contribute to disease and its comorbidities, such as DKD, is not well understood. Here we discuss the role of B cells in the pathogenesis of T1DM and how these cells are activated during disease development. Finally, we speculate on how B cells might contribute to the development of DKD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jeker, L. T., Bour-Jordan, H. & Bluestone, J. A. Breakdown in peripheral tolerance in type 1 diabetes in mice and humans. Cold Spring Harb. Perspect. Med. 2, a007807 (2012).
Bluestone, J. A., Herold, K. & Eisenbarth, G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010).
de Boer, I. H. et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med. 171, 412–420 (2011).
Levine, D. Z. Can rodent models of diabetic kidney disease clarify the significance of early hyperfiltration?: recognizing clinical and experimental uncertainties. Clin. Sci. 114, 109–118 (2008).
Bank, N. Mechanisms of diabetic hyperfiltration. Kidney Int. 40, 792–807 (1991).
Ponchiardi, C., Mauer, M. & Najafian, B. Temporal profile of diabetic nephropathy pathologic changes. Curr. Diab. Rep. 13, 592–599 (2013).
Noorchashm, H. et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J. Immunol. 163, 743–750 (1999).
Serreze, D. V. et al. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 161, 3912–3918 (1998).
Silveira, P. A. et al. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur. J. Immunol. 32, 3657–3666 (2002).
Orban, T. et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 32, 2269–2274 (2009).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Halverson, R., Torres, R. M. & Pelanda, R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 5, 645–650 (2004).
Meffre, E. & Wardemann, H. B-Cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 20, 632–638 (2008).
Casellas, R. et al. Contribution of receptor editing to the antibody repertoire. Science 291, 1541–1544 (2001).
Cambier, J. C., Gauld, S. B., Merrell, K. T. & Vilen, B. J. B-Cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 7, 633–643 (2007).
Duty, J. A. et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med. 206, 139–151 (2009).
Gauld, S. B., Benschop, R. J., Merrell, K. T. & Cambier, J. C. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat. Immunol. 6, 1160–1167 (2005).
Gauld, S. B., Merrell, K. T. & Cambier, J. C. Silencing of autoreactive B cells by anergy: a fresh perspective. Curr. Opin. Immunol. 18, 292–297 (2006).
Merrell, K. T. et al. Identification of anergic B cells within a wild-type repertoire. Immunity 25, 953–962 (2006).
O'Neill, S. K. et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity 35, 746–756 (2011).
Getahun, A., Beavers, N. A., Larson, S. R., Shlomchik, M. J. & Cambier, J. C. Continuous inhibitory signaling by both SHP-1 and SHIP-1 pathways is required to maintain unresponsiveness of anergic B cells. J. Exp. Med. 213, 751–769 (2016).
Getahun, A. et al. Impaired B cell function during viral infections due to PTEN-mediated inhibition of the PI3K pathway. J. Exp. Med. 214, 931–941 (2017).
Cambier, J. C. Autoimmunity risk alleles: hotspots in B cell regulatory signaling pathways. J. Clin. Invest. 123, 1928–1931 (2013).
Akashi, T. et al. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int. Immunol. 9, 1159–1164 (1997).
Xiu, Y. et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in FcγR effector functions. J. Immunol. 180, 2863–2875 (2008).
Hu, C. Y. et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest. 117, 3857–3867 (2007).
Pescovitz, M. D. et al. B-Lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care 37, 453–459 (2014).
Pescovitz, M. D. et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 361, 2143–2152 (2009).
Martin, S. et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N. Engl. J. Med. 345, 1036–1040 (2001).
Jones, J. L. et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc. Natl Acad. Sci. USA 110, 20200–20205 (2013).
Merayo-Chalico, J. et al. Lymphopenia and autoimmunity: a double-edged sword. Hum. Immunol. 77, 921–929 (2016).
Tian, J. et al. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol. 167, 1081–1089 (2001).
Harris, D. P. et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1, 475–482 (2000).
Vehik, K. et al. Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 34, 1897–1901 (2011).
Verge, C. F. et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes 47, 1857–1866 (1998).
Marino, E., Tan, B., Binge, L., Mackay, C. R. & Grey, S. T. B-Cell cross-presentation of autologous antigen precipitates diabetes. Diabetes 61, 2893–2905 (2012).
Hulbert, C., Riseili, B., Rojas, M. & Thomas, J. W. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J. Immunol. 167, 5535–5538 (2001).
Delong, T. et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016).
Babon, J. A. et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat. Med. 22, 1482–1487 (2016).
Wiles, T. A. et al. An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J. Autoimmun. 78, 11–18 (2017).
Barker, J. M. et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J. Clin. Endocrinol. Metab. 89, 3896–3902 (2004).
Sosenko, J. M. et al. The use of electrochemiluminescence assays to predict autoantibody and glycemic progression toward type 1 diabetes in individuals with single autoantibodies. Diabetes Technol. Ther. 19, 183–187 (2017).
Steck, A. K. et al. ECL-IAA and ECL-GADA can identify high-risk single autoantibody-positive relatives in the TrialNet Pathway to Prevention study. Diabetes Technol. Ther. 18, 410–414 (2016).
Wong, F. S. et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 53, 2581–2587 (2004).
DiLillo, D. J. et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol. 180, 361–371 (2008).
Menard, L. et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J. Clin. Invest. 121, 3635–3644 (2011).
Chamberlain, N. et al. Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest. 126, 282–287 (2016).
Panigrahi, A. K. et al. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J. Exp. Med. 205, 2985–2994 (2008).
Smith, M. J. et al. Loss of anergic B cells in prediabetic and new-onset type 1 diabetic patients. Diabetes 64, 1703–1712 (2015).
Huang, S. W., Haedt, L. H., Rich, S. & Barbosa, J. Prevalence of antibodies to nucleic acids in insulin-dependent diabetics and their relatives. Diabetes 30, 873–874 (1981).
Triolo, G. et al. Cross-reactivity of anti-ssDNA antibodies with heparan sulfate in patients with type I diabetes mellitus. Diabetes 38, 718–722 (1989).
Willcox, A., Richardson, S. J., Bone, A. J., Foulis, A. K. & Morgan, N. G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 155, 173–181 (2009).
Leete, P. et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 65, 1362–1369 (2016).
Packard, T. A. et al. B cell receptor affinity for insulin dictates autoantigen acquisition and B cell functionality in autoimmune diabetes. J. Clin. Med. 5, 98 (2016).
Willcox, A. et al. Germinal centre frequency is decreased in pancreatic lymph nodes from individuals with recent-onset type 1 diabetes. Diabetologia 60, 1294–1303 (2017).
Lambert, A. P. et al. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: a population-based study in the United Kingdom. J. Clin. Endocrinol. Metab. 89, 4037–4043 (2004).
Erlich, H. et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes 57, 1084–1092 (2008).
Concannon, P., Rich, S. S. & Nepom, G. T. Genetics of type 1A diabetes. N. Engl. J. Med. 360, 1646–1654 (2009).
Pugliese, A. et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 15, 293–297 (1997).
Cerosaletti, K. & Buckner, J. H. Protein tyrosine phosphatases and type 1 diabetes: genetic and functional implications of PTPN2 and PTPN22. Rev. Diabet. Stud. 9, 188–200 (2012).
Habib, T. et al. Altered B cell homeostasis is associated with type I diabetes and carriers of the PTPN22 allelic variant. J. Immunol. 188, 487–496 (2012).
Dai, X. et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J. Clin. Invest. 123, 2024–2036 (2013).
Simoncic, P. D., Lee-Loy, A., Barber, D. L., Tremblay, M. L. & McGlade, C. J. The T cell protein tyrosine phosphatase is a negative regulator of Janus family kinases 1 and 3. Curr. Biol. 12, 446–453 (2002).
Wiede, F. et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J. Clin. Invest. 121, 4758–4774 (2011).
Long, S. A. et al. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4+ T cells. Genes Immun. 12, 116–125 (2011).
Wiede, F., Sacirbegovic, F., Leong, Y. A., Yu, D. & Tiganis, T. PTPN2-deficiency exacerbates T follicular helper cell and B cell responses and promotes the development of autoimmunity. J. Autoimmun. 76, 85–100 (2017).
Xiao, X. et al. Cellular and humoral immune responses in the early stages of diabetic nephropathy in NOD mice. J. Autoimmun. 32, 85–93 (2009).
Zhang, N. et al. Increased CD4+CXCR5+T follicular helper cells in diabetic nephropathy. Autoimmunity 49, 405–413 (2016).
Atchley, D. H., Lopes-Virella, M. F., Zheng, D., Kenny, D. & Virella, G. Oxidized LDL-anti-oxidized LDL immune complexes and diabetic nephropathy. Diabetologia 45, 1562–1571 (2002).
Nicoloff, G., Blazhev, A., Petrova, C. & Christova, P. Circulating immune complexes among diabetic children. Clin. Dev. Immunol. 11, 61–66 (2004).
Ainsworth, S. K. et al. Diabetic glomerulonephropathy: histopathologic, immunofluorescent, and ultrastructural studies of 16 cases. Hum. Pathol. 13, 470–478 (1982).
Abdelsamie, S. A. et al. Oxidized LDL immune complexes stimulate collagen IV production in mesangial cells via Fc gamma receptors I and III. Clin. Immunol. 139, 258–266 (2011).
Imig, J. D. & Ryan, M. J. Immune and inflammatory role in renal disease. Compr. Physiol. 3, 957–976 (2013).
Saad, A. F., Virella, G., Chassereau, C., Boackle, R. J. & Lopes-Virella, M. F. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J. Lipid Res. 47, 1975–1983 (2006).
Vergani, D., Johnston, C., N., B. A. & Barnett, A. H. Low serum C4 concentrations: an inherited predisposition to insulin dependent diabetes? Br. Med. J. 286, 926–928 (1983).
Barnett, A. H. et al. Low plasma C4 concentrations: association with microangiopathy in insulin dependent diabetes. Br. Med. J. 289, 943–945 (1984).
Duran-Salgado, M. B. & Rubio-Guerra, A. F. Diabetic nephropathy and inflammation. World J. Diabetes 5, 393–398 (2014).
Agrawal, S. & Gupta, S. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J. Clin. Immunol. 31, 89–98 (2011).
Navarro-Gonzalez, J. F., Mora-Fernandez, C., Muros de Fuentes, M. & Garcia-Perez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 7, 327–340 (2011).
Suzuki, Y. et al. Histopathological assessment of renal biopsy specimens of subjects with urine abnormality [Japanese]. Nihon Jinzo Gakkai Shi 37, 284–290 (1995).
Choudhary, N. & Ahlawat, R. S. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran. J. Kidney Dis. 2, 72–79 (2008).
Moriwaki, Y. et al. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 52, 605–608 (2003).
Kalantarinia, K., Awad, A. S. & Siragy, H. M. Urinary and renal interstitial concentrations of TNF-α increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 64, 1208–1213 (2003).
Lund, F. E. Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 20, 332–338 (2008).
Mysliwska, J. et al. High levels of circulating interleukin-10 in diabetic nephropathy patients. Eur. Cytokine Netw. 16, 117–122 (2005).
Peng, X., Xu, J., Wang, P., Zhou, J. & Guo, H. Interleukin-10-1082A/G polymorphism and diabetic nephropathy: a meta-analysis. Med. Sci. Monit. 21, 890–894 (2015).
Gerondakis, S. & Siebenlist, U. Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb. Perspect. Biol. 2, a000182 (2010).
Fiorina, P. et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes 57, 3013–3024 (2008).
Marino, E. et al. CD4+CD25+ T-cells control autoimmunity in the absence of B-cells. Diabetes 58, 1568–1577 (2009).
Zekavat, G. et al. In vivo BLyS/BAFF neutralization ameliorates islet-directed autoimmunity in nonobese diabetic mice. J. Immunol. 181, 8133–8144 (2008).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, discussed the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Germinal centre reaction
-
The anatomical site in which B cells and T cells respond collaboratively to immunogen, leading to B cell proliferation, somatic Ig gene mutation, affinity maturation and immunoglobulin class switch recombination.
- Anergy
-
A mode of B cell tolerance characterized by unresponsiveness to antigenic stimulation, including the inability to become activated, proliferate and secrete antibody.
- Diabetogenic T cells
-
T cells that can cause diabetes, such as insulin-reactive T cells.
- Cross-presenting
-
The process by which antigen presenting cells process and present extracellular antigens to CD8+ T cells.
- High-affinity anti-insulin antibodies
-
Antibodies with an affinity for insulin >10−9 mol/l.
- Plasma cells
-
Terminally differentiated B cells that secrete antibody.
- Recombining sequence rearrangements
-
DNA rearrangements that delete one or both Ig κ genes, leading to expression of Ig λ light chains.
- λ-Immunoglobulin light chain-positive B cells
-
B cells that express λ light chains as a component of their B cell antigen receptor; high levels of these cells is indicative of increased receptor editing.
- Insulitis
-
Inflammation of the pancreas due to infiltration of lymphocytes.
- Haplotypes
-
A set of genes inherited from a single parent.
- Antibody-dependent cell-mediated cytotoxicity
-
The process by which an effector cell of the immune system, such as a natural killer cell, targets a cell for lysis based on the presence of antibodies that are bound to surface antigens on the target cell.
- Mixed meal tolerance test
-
An assay to determine the amount of insulin an individual produces; the individual consumes a drink containing a mixture of protein, fat, and carbohydrates that stimulates the release of insulin from pancreatic β cells; blood is drawn several times over a period of hours and assayed for C-peptide, which reflects endogenous insulin production.
Rights and permissions
About this article
Cite this article
Smith, M., Simmons, K. & Cambier, J. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol 13, 712–720 (2017). https://doi.org/10.1038/nrneph.2017.138
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.138
This article is cited by
-
Common mechanisms underlying diabetic vascular complications: focus on the interaction of metabolic disorders, immuno-inflammation, and endothelial dysfunction
Cell Communication and Signaling (2023)
-
Integrated analysis of RNA methylation regulators crosstalk and immune infiltration for predictive and personalized therapy of diabetic nephropathy
Human Genomics (2023)
-
Increased plasmablasts enhance T cell-mediated beta cell destruction and promote the development of type 1 diabetes
Molecular Medicine (2022)
-
Epigenetic regulation of B cells and its role in autoimmune pathogenesis
Cellular & Molecular Immunology (2022)
-
Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities
Nature Reviews Cancer (2022)