Abstract
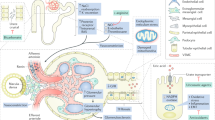
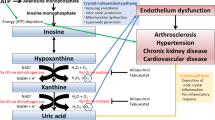
Observational studies have shown that asymptomatic hyperuricemia is associated with increased risks of hypertension, chronic kidney disease (CKD), end-stage renal disease, cardiovascular events, and mortality. Whether these factors represent cause, consequence or incidental associations, however, remains uncertain. Hyperuricemia could be a consequence of impaired kidney function, diuretic therapy or oxidative stress, such that elevated serum urate level represents a marker, rather than a cause, of CKD. On the other hand, small, short-term, single-center studies have shown improvements in blood-pressure control and slowing of CKD progression following serum urate lowering with allopurinol. An adequately powered randomized controlled trial is required to determine whether uric-acid-lowering therapy slows the progression of CKD. This article discusses the rationale for and the feasibility of such a trial. International collaboration is required to plan and conduct a large-scale multicenter trial in order to better inform clinical practice and public health policy about the optimal management of asymptomatic hyperuricemia in patients with CKD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 (2007).
Chadban, S. J. et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J. Am. Soc. Nephrol. 14 (Suppl. 2), 131–138 (2003).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Anavekar, N. S. et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 351, 1285–1295 (2004).
Matsushita, K. et al. Change in estimated GFR associates with coronary heart disease and mortality. J. Am. Soc. Nephrol. 20, 2617–2624 (2009).
Jafar, T. H. et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann. Intern. Med. 135, 73–87 (2001).
Strippoli, G. F., Bonifati, C., Craig, M., Navaneethan, S. D. & Craig, J. C. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database of Systematic Reviews, Issue 4. Art. No.: CD006257. doi: 10.1002/14651858.CD006257 (2006).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Perkovic, V. et al. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: data from the PROGRESS study. J. Am. Soc. Nephrol. 18, 2766–2772 (2007).
Dawson, J., Quinn, T. & Walters, M. Uric acid reduction: a new paradigm in the management of cardiovascular risk? Curr. Med. Chem. 14, 1879–1886 (2007).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359, 1811–1821 (2008).
Domrongkitchaiporn, S. et al. Risk factors for development of decreased kidney function in a southeast Asian population: a 12-year cohort study. J. Am. Soc. Nephrol. 16, 791–799 (2005).
Hsu, C. Y., Iribarren, C., McCulloch, C. E., Darbinian, J. & Go, A. S. Risk factors for end-stage renal disease: 25-year follow-up. Arch. Intern. Med. 169, 342–350 (2009).
Iseki, K. et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am. J. Kidney Dis. 44, 642–650 (2004).
Obermayr, R. P. et al. Elevated uric acid increases the risk for kidney disease. J. Am. Soc. Nephrol. 19, 2407–2413 (2008).
Weiner, D. E. et al. Uric acid and incident kidney disease in the community. J. Am. Soc. Nephrol. 19, 1204–1211 (2008).
Chonchol, M. et al. Relationship of uric acid with progression of kidney disease. Am. J. Kidney Dis. 50, 239–247 (2007).
Rosolowsky, E. T. et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin. J. Am. Soc. Nephrol. 3, 706–713 (2008).
Sturm, G., Kollerits, B., Neyer, U., Ritz, E. & Kronenberg, F. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp. Gerontol. 43, 347–352 (2008).
Syrjänen, J., Mustonen, J. & Pasternack, A. Hypertriglyceridaemia and hyperuricemia are risk factors for progression of IgA nephropathy. Nephrol. Dial. Transplant. 15, 34–42 (2000).
Myllymäki, J. et al. Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol. Dial. Transplant. 20, 89–95 (2005).
Tang, Z., Cheng, L. T., Li, H. Y. & Wang, T. Serum uric acid and endothelial dysfunction in continuous ambulatory peritoneal dialysis patients. Am. J. Nephrol. 29, 368–373 (2009).
Park, J. T. et al. Uric acid is associated with the rate of residual renal function decline in peritoneal dialysis patients. Nephrol. Dial. Transplant. 24, 3520–3525 (2009).
Madero, M. et al. Uric acid and long-term outcomes in CKD. Am. J. Kidney Dis. 53, 796–803 (2009).
Suliman, M. E. et al. J-shaped mortality relationship for uric acid in CKD. Am. J. Kidney Dis. 48, 761–771 (2006).
Lee, S. M. et al. Low serum uric acid level is a risk factor for death in incident hemodialysis patients. Am. J. Nephrol. 29, 79–85 (2009).
Navaneethan, S. D. & Beddhu, S. Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol. Dial. Transplant. 24, 1260–1266 (2009).
Armstrong, K. A., Johnson, D. W., Campbell, S. B., Isbel, N. M. & Hawley, C. M. Does uric acid have a pathogenetic role in graft dysfunction and hypertension in renal transplant recipients? Transplantation 80, 1565–1571 (2005).
Haririan, A. et al. The independent association between serum uric acid and graft outcomes after kidney transplantation. Transplantation 89, 573–579 (2010).
Akalin, E., Ganeshan, S. V., Winston, J. & Muntner, P. Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation 86, 652–658 (2008).
Doehner, W. et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 105, 2619–2624 (2002).
Guthikonda, S., Sinkey, C., Barenz, T. & Haynes, W. G. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 107, 416–421 (2003).
Feig, D. I., Soletsky, B. & Johnson, R. J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 300, 924–932 (2008).
Shelmadine, B., Bowden, R. G., Wilson, R. L., Beavers, D. & Hartman, J. The effects of lowering uric acid levels using allopurinol on markers of metabolic syndrome in end-stage renal disease patients: a pilot study. Anadolu. Kardiyol. Derg. 9, 385–389 (2009).
Neal, D. A., Tom, B. D., Gimson, A. E., Gibbs, P. & Alexander, G. J. Hyperuricemia, gout, and renal function after liver transplantation. Transplantation 72, 1689–1691 (2001).
Siu, Y. P., Leung, K. T., Tong, M. K. & Kwan, T. H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 47, 51–59 (2006).
Goicoechea, M. et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 5, 1388–1393 (2010).
Kao, M. P., Ang, D. S. & Struthers, A. D. Allopurinol reduces both left ventricular hypertrophy and endothelial dysfunction in patients with chronic kidney disease. Presented at the XLVII ERA-EDTA Congress, Munich, Germany (2010).
Sanchez-Lozada, L. G. et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol. 108, 69–78 (2008).
Acknowledgements
We are grateful to C. Thompson, a Biostatistician at the Australasian Kidney Trials Network, University of Queensland, Brisbane, Qld 4102, Australia, for his invaluable statistical assistance.
Author information
Authors and Affiliations
Contributions
S. V. Badve, F. Brown, J. Kanellis, G. K. Rangan, V. Perkavic contributed equally to researching data for the article, writing and reviewing the manuscript before submission. C. M. Hawley made a substantial contribution to discussion of content, writing and review of the manuscript. D. W. Johnson was involved in writing and review of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Summary of studies that have examined the relationship between uric acid level and various clinical outcomes (DOC 70 kb)
Rights and permissions
About this article
Cite this article
Badve, S., Brown, F., Hawley, C. et al. Challenges of conducting a trial of uric-acid-lowering therapy in CKD. Nat Rev Nephrol 7, 295–300 (2011). https://doi.org/10.1038/nrneph.2010.186
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.186
This article is cited by
-
Association between dietary intake of flavonoids and hyperuricemia: a cross-sectional study
BMC Public Health (2023)
-
Treatment of asymptomatic hyperuricemia complicated by renal damage: a controversial issue
International Urology and Nephrology (2019)
-
Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta-analysis
BMC Nephrology (2015)
-
Factors associated with chronic musculoskeletal pain in patients with chronic kidney disease
BMC Nephrology (2014)
-
Establishing a clinical trials network in nephrology: experience of the Australasian Kidney Trials Network
Kidney International (2014)