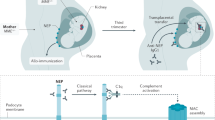
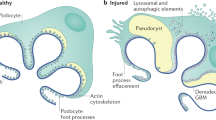
Abstract
The podocytopathies, including minimal-change nephropathy, focal segmental glomerulosclerosis, collapsing glomerulopathy, and diffuse mesangial sclerosis, involve diverse types of injury to podocytes. These injuries can have genetic causes, or can be caused by viral infection, mechanical stress, medication or—probably—immunologic injury. Several lines of evidence—including the immunosuppressive effects of standard therapies—suggest a role for immunologic injury in some cases, but the precise pathologic mechanisms are far from clear. Despite this uncertainty, newly available biologic therapies that target immune cells and cytokines have been used to treat a number of patients with different podocytopathies. Of these therapies, the greatest experience has been gained with rituximab. The data on all such therapies remain too fragmentary to provide firm conclusions, but further clinical research with such agents might help to define pathogenetic pathways and could potentially contribute to new therapies.
Key Points
-
The immunopathogenesis of idiopathic podocyte disease remains uncertain
-
Current immunosuppressive treatments for podocytopathies are nonspecific and potentially toxic
-
Monoclonal antibodies might help to elucidate the pathogenesis of podocytopathies and might also be useful for treatment of these diseases
-
At present, the use of monoclonal antibodies other than rituximab to treat podocytopathies has been very limited
-
Rituximab therapy has been associated with remission in cases of minimal-change nephropathy and focal segmental glomerulosclerosis
-
Occasional reports have shown remission of idiopathic nephrotic syndrome following treatment with tumor necrosis factor antagonists
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Mundel, P. & Shankland, S. J. Podocyte biology and response to injury. J. Am. Soc. Nephrol. 13, 3005–3015 (2002).
Pavenstädt, H., Kriz, W. & Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 83, 253–307 (2003).
Schnaper, H. et al. Nephrotic syndrome: minimal change disease, focal segmental glomerulosclerosis, and collapsing glomerulopathy, in Disease of the Kidney and Urinary Tract, 8th edn (Lippincott Williams & Wilkins, Philadelphia, 2006).
Barisoni, L., Schnaper, H. W. & Kopp, J. B. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin. J. Am. Soc. Nephrol. 2, 529–542 (2007).
Shalhoub, R. J. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 2, 556–560 (1974).
Koenecke, C., Ukena, S. N., Ganser, A. & Franzke, A. Regulatory T cells as therapeutic target in Hodgkin's lymphoma. Expert Opin. Ther. Targets 12, 769–782 (2008).
Mathieson, P. W. Minimal change nephropathy and focal segmental glomerulosclerosis. Semin. Immunopathol. 29, 415–426 (2007).
Reiser, J. et al. Induction of B7–1 in podocytes is associated with nephrotic syndrome. J. Clin. Invest. 113, 1390–1397 (2004).
Yamada, A., Salama, A. D. & Sayegh, M. H. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J. Am. Soc. Nephrol. 13, 559–575 (2002).
Lai, K. W. et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J. Am. Soc. Nephrol. 18, 1476–1485 (2007).
Karras, A. et al. Renal and thymic pathology in thymoma-associated nephropathy: report of 21 cases and review of the literature. Nephrol. Dial. Transplant. 20, 1075–1082 (2005).
Ronco, P. & Debiec, H. Pathophysiological lessons from rare associations of immunological disorders. Pediatr. Nephrol. 24, 3–8 (2008).
Lapillonne, H. et al. Stem cell mobilization in idiopathic steroid-sensitive nephrotic syndrome. Pediatr. Nephrol. 23, 1251–1256 (2008).
Araya, C. E. et al. A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr. Nephrol. 21, 603–610 (2006).
Sharma, M., Sharma, R., Reddy, S. R., McCarthy, E. T. & Savin, V. J. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation 73, 366–372 (2002).
Zimmerman, S. W. Increased urinary protein excretion in the rat produced by serum from a patient with recurrent focal glomerular sclerosis after renal transplantation. Clin. Nephrol. 22, 32–38 (1984).
Koyama, A., Fujisaki, M., Kobayashi, M., Igarashi, M. & Narita, M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 40, 453–460 (1991).
Dantal, J. et al. Effect of plasma protein adsorption on protein excretion in kidney-transplant recipients with recurrent nephrotic syndrome. N. Engl. J. Med. 330, 7–14 (1994).
Le Berre, L. et al. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J. Clin. Invest. 109, 491–498 (2002).
Le Berre, L. et al. Renal macrophage activation and TH2 polarization precedes the development of nephrotic syndrome in Buffalo/Mna rats. Kidney Int. 68, 2079–2090 (2005).
Ali, A. A. et al. Minimal-change glomerular nephritis. Normal kidneys in an abnormal environment? Transplantation 58, 849–852 (1994).
Savin, V. J. et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N. Engl. J. Med. 334, 878–883 (1996).
Ghiggeri, G. M., Carraro, M. & Vincenti, F. Recurrent focal glomerulosclerosis in the era of genetics of podocyte proteins: theory and therapy. Nephrol. Dial. Transplant. 19, 1036–1040 (2004).
Carraro, M. et al. Nephrotic urine prevents increased rat glomerular albumin permeability induced by serum from the same patient with idiopathic nephrotic syndrome. Nephrol. Dial. Transplant. 18, 689–693 (2003).
Sharma, R., Sharma, M., McCarthy, E. T., Ge, X. L. & Savin, V. J. Components of normal serum block the focal segmental glomerulosclerosis factor activity in vitro. Kidney Int. 58, 1973–1979 (2000).
Kemper, M. J., Wolf, G. & Müller-Wiefel, D. E. Transmission of glomerular permeability factor from a mother to her child. N. Engl. J. Med. 344, 386–387 (2001).
Kemper, M. J., Meyer-Jark, T., Lilova, M. & Müller-Wiefel, D. E. Combined T- and B-cell activation in childhood steroid-sensitive nephrotic syndrome. Clin. Nephrol. 60, 242–247 (2003).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Daniel, V., Trautmann, Y., Konrad, M., Nayir, A. & Schärer, K. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin. Nephrol. 47, 289–297 (1997).
Bagga, A., Vasudev, A. S., Moudgil, A. & Srivastava, R. N. Peripheral blood lymphocyte subsets in idiopathic nephrotic syndrome of childhood. Indian J. Med. Res. 104, 292–295 (1996).
Pawluczyk, I. Z. & Harris, K. P. Macrophages promote prosclerotic responses in cultured rat mesangial cells: a mechanism for the initiation of glomerulosclerosis. J. Am. Soc. Nephrol. 8, 1525–1536 (1997).
Lin, C. Y. & Chien, J. W. Increased interleukin-12 release from peripheral blood mononuclear cells in nephrotic phase of minimal change nephrotic syndrome. Acta Paediatr. Taiwan 45, 77–80 (2004).
Nishimura, M. et al. Focal segmental glomerular sclerosis, a type of intractable chronic glomerulonephritis, is a stem cell disorder. J. Exp. Med. 179, 1053–1058 (1994).
Yoshida, F., Matsuo, S., Fujishima, H., Kim, H. K. & Tomita, T. Renal lesions of the FGS strain of mice: a spontaneous animal model of progressive glomerulosclerosis. Nephron 66, 317–325 (1994).
Humphreys, B. D., Vanguri, V. K., Henderson, J. & Antin, J. H. Minimal-change nephrotic syndrome in a hematopoietic stem-cell transplant recipient. Nat. Clin. Pract. Nephrol. 2, 535–539 (2006).
Heras, M. et al. Nephrotic syndrome resulting from focal segmental glomerulosclerosis in a peripheral blood stem cell transplant patient. J. Nephrol. 20, 495–498 (2007).
Colombo, A. A. et al. Nephrotic syndrome after allogeneic hematopoietic stem cell transplantation as a late complication of chronic graft-versus-host disease. Transplantation 81, 1087–1092 (2006).
Sellier-Leclerc, A. L. et al. A humanized mouse model of idiopathic nephrotic syndrome suggests a pathogenic role for immature cells. J. Am. Soc. Nephrol. 18, 2732–2739 (2007).
FDA Approved Drug Products [online].
World Health Organization International Nonproprietary Names program [online].
Hudson, P. J. & Souriau, C. Engineered antibodies. Nat. Med. 9, 129–134 (2003).
Mottershead, M. & Neuberger, J. Daclizumab. Expert Opin. Biol. Ther. 7, 1583–1596 (2007).
Church, A. C. Clinical advances in therapies targeting the interleukin-2 receptor. QJM 96, 91–102 (2003).
Francois, H., Daugas, E., Bensman, A. & Ronco, P. Unexpected efficacy of rituximab in multirelapsing minimal change nephrotic syndrome in the adult: first case report and pathophysiological considerations. Am. J. Kidney Dis. 49, 158–161 (2007).
Park, S. S., Hahn, W. H., Kim, S. D. & Cho B. S. Remission of refractory minimal change nephrotic syndrome after basiliximab therapy. Pediatric. Nephrol. doi:10.1007/S00467-009-1145-6.
Salama, A. D. & Pusey, C. D. Drug insight: rituximab in renal disease and transplantation. Nat. Clin. Pract. Nephrol. 2, 221–230 (2006).
Ruggenenti, P. et al. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J. Am. Soc. Nephrol. 14, 1851–1857 (2003).
Pescovitz, M. D., Book, B. K. & Sidner, R. A. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N. Engl. J. Med. 354, 1961–1963 (2006).
Kamar, N. et al. Treatment of focal segmental glomerular sclerosis with rituximab: 2 case reports. Clin. Nephrol. 67, 250–254 (2007).
Gossmann, J. et al. Abrogation of nephrotic proteinuria by rituximab treatment in a renal transplant patient with relapsed focal segmental glomerulosclerosis. Transpl. Int. 20, 558–562 (2007).
Hristea, D. et al. Successful treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation by plasmapheresis and rituximab. Transpl. Int. 20, 102–105 (2007).
Meyer, T. N., Thaiss, F. & Stahl, R. A. Immunoadsorbtion and rituximab therapy in a second living-related kidney transplant patient with recurrent focal segmental glomerulosclerosis. Transpl. Int. 20, 1066–1071 (2007).
Apeland, T. & Hartmann, A. Rituximab therapy in early recurrent focal segmental sclerosis after renal transplantation. Nephrol. Dial. Transplant. 23, 2091–2094 (2008).
Bayrakci, U. S., Baskin, E., Sakalli, H., Karakayali, H. & Haberal, M. Rituximab for posttransplant recurrences of FSGS. Pediatr. Transplant. 13, 240–243 (2008).
Yabu, J. M., Ho, B., Scandling, J. D. & Vincenti, F. Rituximab failed to improve nephrotic syndrome in renal transplant patients with recurrent focal segmental glomerulosclerosis. Am. J. Transplant. 8, 222–227 (2008).
Marks, S. D. & McGraw, M. Does rituximab treat recurrent focal segmental glomerulosclerosis postrenal transplantation? Pediatr. Nephrol. 22, 158–160 (2007).
El-Firjani, A. et al. Post-transplant focal segmental glomerulosclerosis refractory to plasmapheresis and rituximab therapy. Nephrol. Dial. Transplant. 23, 425 (2008).
Nozu, K. et al. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr. Nephrol. 20, 1660–1663 (2005).
Guigonis, V. et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr. Nephrol. 23, 1269–1279 (2008).
Bagga, A., Sinha, A. & Moudgil, A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N. Engl. J. Med. 356, 2751–2752 (2007).
Nakayama, M. et al. Rituximab for refractory focal segmental glomerulosclerosis. Pediatr. Nephrol. 23, 481–485 (2008).
Benz, K. et al. Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr. Nephrol. 19, 794–797 (2004).
Gilbert, R. D., Dötsch, J., Rascher, W. & Stachel, D. Rituximab therapy for steroid-dependent minimal change nephrotic syndrome. Pediatr. Nephrol. 21, 1698–1700 (2006).
Hofstra, J. M., Deegens, J. K. & Wetzels, J. F. Rituximab: effective treatment for severe steroid-dependent minimal change nephrotic syndrome? Nephrol. Dial. Transplant. 22, 2100–2102 (2007).
Smith, G. C. Is there a role for rituximab in the treatment of idiopathic childhood nephrotic syndrome? Pediatr. Nephrol. 22, 893–898 (2007).
Suri, M., Tran, K., Sharma, A. P., Filler, G. & Grimmer, J. Remission of steroid-resistant nephrotic syndrome due to focal and segmental glomerulosclerosis using rituximab. Int. Urol. Nephrol. 40, 807–810 (2008).
Yang, T., Nast, C. C., Vo, A. & Jordan, S. C. Rapid remission of steroid and mycophenolate mofetil (MMF)-resistant minimal change nephrotic syndrome after rituximab therapy. Nephrol. Dial. Transplant. 23, 377–380 (2008).
Gray, M., Miles, K., Salter, D., Gray, D. & Savill, J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc. Natl Acad. Sci. USA 104, 14080–14085 (2007).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Taube, D., Brown, Z. & Williams, D. G. Impaired lymphocyte and suppressor cell function in minimal change nephropathy, membranous nephropathy and focal glomerulosclerosis. Clin. Nephrol. 22, 176–182 (1984).
Calabrese, L. H. & Molloy, E. S. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann. Rheum. Dis. 67 (Suppl. 3), iii64–iii65 (2008).
Waldmann, H. & Hale, G. CAMPATH: from concept to clinic. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 360, 1707–1711 (2005).
Buttmann, M. & Rieckmann, P. Treating multiple sclerosis with monoclonal antibodies. Expert Rev. Neurother. 8, 433–455 (2008).
Pascual, J. et al. Alemtuzumab induction and recurrence of glomerular disease after kidney transplantation. Transplantation 83, 1429–1434 (2007).
Ciancio, G. & Burke, G. W. 3rd. Alemtuzumab (Campath-1H) in kidney transplantation. Am. J. Transplant. 8, 15–20 (2008).
Noris, M. et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J. Am. Soc. Nephrol. 18, 1007–1018 (2007).
Clatworthy, M. R. et al. Anti-glomerular basement membrane disease after alemtuzumab. N. Engl. J. Med. 359, 768–769 (2008).
Walsh, M., Wallin, E. F. & Jayne, D. R. Long-term follow-up of relapsing/refractory ANCA associated vasculitis treated with the lymphocyte depleting antibody alemtuzumab (CAMPATH-1H) [abstract]. J. Am. Soc. Nephrol. 18, 48A (2007).
Vincenti, F. Costimulation blockade in autoimmunity and transplantation. J. Allergy Clin. Immunol. 121, 299–306 (2008).
Kitching, A. R., Huang, X. R., Ruth, A. J., Tipping, P. G. & Holdsworth, S. R. Effects of CTLA4-Fc on glomerular injury in humorally mediated glomerulonephritis in BALB/c mice. Clin. Exp. Immunol. 128, 429–435 (2002).
Reynolds, J. et al. CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J. Clin. Invest. 105, 643–651 (2000).
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117, 244–279 (2008).
Rigby, W. F. Drug insight: different mechanisms of action of tumor necrosis factor antagonists—passive-aggressive behavior? Nat. Clin. Pract. Rheumatol. 3, 227–233 (2007).
Ehrenstein, M. R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285 (2004).
Suranyi, M. G., Guasch, A., Hall, B. M. & Myers, B. D. Elevated levels of tumor necrosis factor-α in the nephrotic syndrome in humans. Am. J. Kidney Dis. 21, 251–259 (1993).
Raveh, D., Shemesh, O., Ashkenazi, Y. J., Winkler, R. & Barak, V. Tumor necrosis factor-α blocking agent as a treatment for nephrotic syndrome. Pediatr. Nephrol. 19, 1281–1284 (2004).
Leroy, S. et al. Tumor necrosis factor-α blocking agent as a treatment for steroid resistant nephrotic syndrome [abstract]. J. Am. Soc. Nephrol. 18, 565A (2007).
Ricklin, D. & Lambris, J. D. Complement-targeted therapeutics. Nat. Biotechnol. 25, 1265–1275 (2007).
Cunningham, P. N. & Quigg, R. J. Contrasting roles of complement activation and its regulation in membranous nephropathy. J. Am. Soc. Nephrol. 16, 1214–1222 (2005).
Appel, G. et al. Eculizumab (C5 complement inhibitor) in the treatment of idiopathic membranous nephropathy: preliminary baseline and pharmacokinetic (PK)/pharmacodynamic (PD) data [abstract]. J. Am. Soc. Nephrol. 13, 668A (2002).
Turnberg, D. et al. Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J. Immunol. 177, 4094–4102 (2006).
Rangan, G. K., Pippin, J. W. & Couser, W. G. C5b-9 regulates peritubular myofibroblast accumulation in experimental focal segmental glomerulosclerosis. Kidney Int. 66, 1838–1848 (2004).
Abbate, M. et al. Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J. Am. Soc. Nephrol. 19, 1158–1167 (2008).
Morita, Y. et al. Complement activation products in the urine from proteinuric patients. J. Am. Soc. Nephrol. 11, 700–707 (2000).
Gagliardini, E. & Benigni, A. Therapeutic potential of TGF-β inhibition in chronic renal failure. Expert Opin. Biol. Ther. 7, 293–304 (2007).
Wahab, N. A. & Mason, R. M. A critical look at growth factors and epithelial-to-mesenchymal transition in the adult kidney. Interrelationships between growth factors that regulate EMT in the adult kidney. Nephron Exp. Nephrol. 104, e129–e134 (2006).
Abbate, M. et al. Transforming growth factor-β1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: a central pathway in progressive glomerulosclerosis. Am. J. Pathol. 161, 2179–2193 (2002).
Ma, L. J. et al. Divergent effects of low versus high dose anti-TGF-β antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int. 65, 106–115 (2004).
Kasuga, H. et al. Effects of anti-TGF-β type II receptor antibody on experimental glomerulonephritis. Kidney Int. 60, 1745–1755 (2001).
Dornhöfer, N. et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 66, 5816–5827 (2006).
Ikawa, Y. et al. Neutralizing monoclonal antibody to human connective tissue growth factor ameliorates transforming growth factor-β-induced mouse fibrosis. J. Cell. Physiol. 216, 680–687 (2008).
Acknowledgements
The authors would like to thank Dr. Paolo Cravedi and Dr. Meryl Waldman for critical reading of the manuscript. Our work is supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Marasà, M., Kopp, J. Monoclonal antibodies for podocytopathies: rationale and clinical responses. Nat Rev Nephrol 5, 337–348 (2009). https://doi.org/10.1038/nrneph.2009.70
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.70
This article is cited by
-
Angiotensin II induces tumor necrosis factor-α expression and release from cultured human podocytes
Inflammation Research (2012)
-
Pathogenesis and therapy of focal segmental glomerulosclerosis: an update
Pediatric Nephrology (2011)