Abstract
Probiotics and prebiotics are increasingly being added to foodstuffs with claims of health benefits. Probiotics are live microorganisms that are thought to have beneficial effects on the host, whereas prebiotics are ingredients that stimulate the growth and/or function of beneficial intestinal microorganisms. But can these products directly modulate immune function and influence inflammatory diseases? Here, Nature Reviews Immunology asks four experts to discuss these issues and provide their thoughts on the future application of probiotics as a disease therapy.
Similar content being viewed by others
Do probiotics and prebiotics modulate immune function? And if so, how?
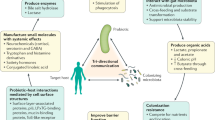
Michiel Kleerebezem. The maintenance of intestinal immune and metabolic homeostasis in mammals is strongly affected by the interactions between the mucosa and the intestinal microbiota1. The positive effects of prebiotics and probiotics on human health have frequently been attributed to their indirect and direct immunomodulating capacity, although other mechanisms of action have also been proposed, such as modulation of cellular metabolism, epithelial barrier functions or proliferation. Nevertheless, human studies using prebiotic and probiotic interventions to induce immune-health benefits — including the suppression of allergic and autoimmune disease or the stimulation of immune defence — have generated contradictory results. These contradictory results may in part be due to differences in study design, but they are also due to our lack of understanding of the specificity and mechanisms by which these prebiotics and probiotics, delivered in either supplements or foods, elicit their effects. For example, it remains largely unknown to what extent prebiotic compounds may directly affect immune signalling pathways, or whether they act exclusively via their modulation of the endogenous intestinal microbiota. Probiotics may elicit immunomodulatory effects through direct interactions with the intestinal epithelium, especially in the small intestine, which is less densely populated by the commensal microbiota2. By contrast, probiotic immunomodulatory effects in the densely populated colon are more likely to occur via modulation of the endogenous microbiota3.
Several probiotic effector molecules involved in immune interactions have been identified, including bacterial cell wall components such as peptidoglycan and lipoteichoic acid, as well as specific proteins (reviewed in Refs 4, 72). For some of these effector molecules, their modes of action on host immune responses have been described and involve the modulation of several receptor signalling cascades that are known to have a prominent role in the regulation of the human immune system (reviewed in Ref. 4). However, these mechanistic studies are generally based on in vitro cell-culture models and may not accurately reflect the in vivo situation. Importantly, the probiotic products that are currently on the market predominantly target the healthy population with the claim to prophylactically reduce disease risk, rather than to treat disease or provide a therapeutic benefit. Consequently, immune-health benefits should be measured in healthy individuals, and validation of the prophylactic health effects would benefit from challenge models in which the immune system of the consumer is subjected to a (controlled) stimulus to allow the quantitative evaluation of the proposed prophylactic effect.
Recent in vivo studies in healthy human volunteers measured the changes in gene transcription profiles to determine the molecular responses that occur in the human duodenal mucosa following consumption of probiotic Lactobacillus spp.5,6. These nutrigenomic studies showed that the mucosal responses to distinct lactobacilli are profoundly different, illustrating the specificity of the host responses to specific bacterial strains and/or species6, or even different preparations of the same bacterial strain5. The same transcriptional responses were consistently detected in all participating volunteers. These responses represented biologically coherent responses and predicted strain-specific consequences on mucosal immune function that were congruent with the physiological effects measured in animal and human studies using these probiotic lactobacilli. Therefore, these studies provide in vivo support for strain- and species-specific immunomodulatory capacities of distinct probiotic lactobacilli. Such immunomodulation ranges from immune tolerance induced by Lactobacillus plantarum5 to stimulation of innate and T helper 1 (TH1)-type immune responses by Lactobacillus acidophilus and modulation of the TH1/TH2 response balance by Lactobacillus casei6. These findings illustrate mechanisms by which probiotics can modulate immune-related responses in the mucosa of the intestine and thereby influence mucosal defences.
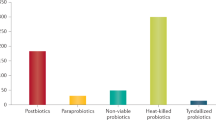
Maria Rescigno. Microorganisms capable of imparting beneficial effects on human health are collectively called probiotics. However, as it is very difficult to demonstrate a beneficial effect on an already healthy individual, it has become common to term any seemingly harmless microorganism isolated from the human gut as a probiotic. In addition, there is the misconception that different probiotics will have similar effects on the immune system. However, it is becoming evident that completely different effects may be observed depending on the species and the strain of the microorganism used. Some strains have a pro-inflammatory effect, whereas others are more anti-inflammatory7. Probiotics may also have indirect immunomodulatory functions through their actions on non-immune cells such as epithelial cells, and may even exert their effect independently of the immune system by inhibiting the colonization of the intestinal mucosa by pathogenic microorganisms and/or by inducing the release of antimicrobial peptides. Some of these activities may be mediated by structure-associated compounds, whereas others are mediated by metabolic products, which we have termed postbiotics8.
The active components of probiotics have only recently started to be unravelled. For instance, the cell surface-associated exopolysaccharide of the probiotic Bifidobacterium breve reduces the production of pro-inflammatory cytokines and suppresses the generation of B. breve-specificantibodies, thus allowing this probiotic to be tolerated in the gut9. Furthermore, exopolysaccharide also impairs the persistence of the pathogen Citrobacter rodentium. The secreted protein p40 of Lactobacillus rhamnosus GG has been shown to activate the epidermal growth factor receptor (EGFR) pathway, thereby reducing cytokine-induced epithelial cell apoptosis and protecting against experimental colitis10. Lactocepin, a protease secreted by Lactobacillus paracasei, degrades some pro-inflammatory chemokines, including CXC-chemokine ligand 10 (CXCL10), inhibits the recruitment of inflammatory cells into the mucosal tissue and protects against colitis in mice11. Lactobacillus brevis-secreted polyphosphate has protective effects on epithelial cells via the activation of the mitogen-activated protein kinase p38 (Ref. 12). Other, not yet identified compounds secreted by Faecalibacterium prausnitzii and L. paracasei can inhibit nuclear factor-κB (NF-κB) activation and protect against experimental colitis or ongoing inflammation in tissues from patients with inflammatory bowel disease (IBD)8,13. Hence, probiotics may have disparate functions and protect the gut barrier via immune- and non-immune-mediated effects.
Todd R. Klaenhammer. It has been long demonstrated that oral delivery of probiotic microorganisms can modulate immune function. Early research showed that feeding L. acidophilus and bifidobacteria in a fermented milk product to human volunteers resulted in significant elevations of total IgA, and specifically in the levels of IgA specific for Salmonella enterica subsp. enterica serovar Typhi14. Subsequently, it was established that different strains of lethally irradiated lactobacilli can differentially activate mouse dendritic cells (DCs), notably with substantial differences between strains in the ability to induce interleukin-12 (IL-12) and tumour necrosis factor15.
Multiple probiotic cultures are now routinely delivered orally as dietary supplements or in fermented dairy foods at levels exceeding 108 colony forming units per gram (cfu g−1). Flooding the intestinal mucosa with relatively large concentrations of probiotic microorganisms, compared with the low concentration of resident microbiota in the upper small intestine (∼103–107 cfu g−1), certainly offers significant potential to affect the immunological responses of the host. Today, it is well established that cell-surface proteins16, lipoteichoic acid17,18, peptidoglycan-dervived muropeptides19, exopolysaccharides20 and pili-type structures21,22,23 exert immunological responses from a variety of immune cells, including DCs, macrophages and lymphocytes. Alterations of these bacterial structures in terms of their expression, amino acid sequence, charge and glycosylation patterns can elicit significant changes in their recognition by both intestinal epithelial cells (IECs) and DCs24.
Matthias Volkmar Kopp. There is little doubt that our immune system is connected through intensive crosstalk with the human ecosystem, which hosts approximately 1013–1014 bacteria25. However, prebiotics and probiotics are umbrella terms, and different prebiotics and different genera, species and even strains of probiotics might have different effects on the immune system. Furthermore, the exact mechanisms of the immunomodulatory effects of prebiotics and probiotics have not been fully elucidated.
There are several lines of evidence suggesting that probiotics exert immune modulation via their interaction with IECs and DCs in the gastrointestinal tract. Several Lactobacillus spp. have been shown to change the phenotype of DCs and their cytokine patterns. Moreover, the induction of regulatory T (TReg) cells, the upregulation of IL-10 and transforming growth factor-β (TGFβ) and an increase in local IgA production have been observed26. Recently, it has been proposed that in mice the acetate produced by protective bifidobacteria improves intestinal defence by inhibiting the translocation of pathogenic bacteria from the gut lumen to the blood, and thereby blocking subsequent infection27.
However, it is arguable whether the in vitro data and results from animal models are transferable to humans. We observed that L. rhamnosus GG could upregulate IL-10 and interferon-γ (IFNγ) expression levels in isolated mononuclear cells from healthy individuals, irrespective of whether they had received L. rhamnosus GG or a placebo before blood cell isolation28. However, despite the immunomodulatory effects of L. rhamnosus GG in this in vitro study, diet supplementation with L. rhamnosus GG during pregnancy and early infancy had no discernable effect on clinical end points such as the development of atopic eczema or the severity of the disease in affected children29.
Considering that the gastrointestinal tract is the primary target organ of probiotics, a description of the interrelationships between the resident microbial flora, the antigen load and the integrity of the epithelium might help to determine the potential of specific supplementation with prebiotics or probiotics. However, to date, it remains unclear whether the immunomodulatory effects of probiotics are short term or are sustained and/or reproducible. Finally, in vitro studies have often used artificial culture conditions (for example, heat-killed probiotic strains or added antibiotics) because the exponential growth of live bacteria would exhaust culture conditions and induce cell necrosis; however, these non-physiological conditions hamper a comparison with the effects of probiotics in humans.
Can we expect prebiotics and probiotics to elicit immune effects in (all) healthy subjects?
M.R. As mentioned above, probiotics can have pro-inflammatory or anti-inflammatory properties. Hence, the choice of using one strain over another in healthy individuals is quite arbitrary. What is the desired effect? Who decides whether an individual would benefit most from one immunomodulatory property versus another? In addition, if it is difficult to observe the biological effects of a probiotic in the unperturbed state, how can its activity be evaluated? Some immunological markers could be assessed. For instance, administration of Bifidobacterium infantis 35624 in healthy volunteers has been shown to increase the amount of IL-10 produced by peripheral blood mononuclear cells and the expression of the transcription factor forkhead box P3 (FOXP3) by TReg cells30.
The variability of the response to a probiotic strain is quite large among individuals30, and it is difficult to predict what the biological effects of these changes will be in the absence of an immunological challenge. The beneficial effects may range from reducing the risks of allergic reactions and inflammatory conditions to protection from infectious agents. As most of the probiotics are incapable of colonizing the gut and are eliminated shortly after consumption, their biological effects may be lost when the bacteria are no longer administered, and it is not clear what would be the outcome of prolonged administration of an anti-inflammatory strain. Will it weaken the immune response? In addition, as the L. rhamnosus GG-secreted soluble protein p40 triggers the EGFR pathway10, could it have detrimental effects in individuals with a family history of epithelial cancers?
Prebiotics can favour the preferential growth of some bacterial strains present in the microbiota. The recent findings that individuals can be subdivided into 'enterotypes' according to their microbiota31 and that these may be predictive of susceptibility to disease and may be modulated by the diet32 makes the world of prebiotics particularly interesting as a tool to modify the enterotype of an individual. However, until an enterotype that is associated with the 'healthy' condition is clearly identified, it is arbitrary to use one prebiotic rather than another.
One important question is whether all probiotics are harmless in healthy individuals. We showed that L. plantarum v299, which is a potent pro-inflammatory strain of probiotic, can have detrimental effects on healthy intestinal human tissues, such as crypt destruction and the recruitment of inflammatory cells8, and can worsen experimental colitis if given to mice before administration of dextran sulphate sodium (DSS)33.
Hence, inflammatory strains of probiotics may be harmful. They may nevertheless be used not as probiotics but as vectors for vaccine development or as adjuvants to improve the activation of the immune response. Before proposing a probiotic or prebiotic for use in healthy individuals, its immunomodulatory properties should be thoroughly evaluated in reliable model systems and individual probiotic strains should be carefully considered according to the desired effect.
T.R.K. The inherent variability of 'healthy subjects' would certainly result in diverse immunological responses across the treatment group. There are many studies in which probiotic microorganisms have been fed to animals and humans, and immunological responses measured. Notably among these are the reports by van Baarlen et al.5,6 that evaluated the in vivo human mucosal transcriptome responses to different species of lactobacilli and indicated how probiotics may modulate human cellular pathways. Interestingly, they found that the transcriptomes clustered most closely within each individual subject, and the variation in gene expression was largest between different individuals, rather than between different probiotic strains. Immunological responses would be anticipated in healthy subjects following probiotic consumption, particularly for those probiotic microorganisms that elicit dominant pro-inflammatory or anti-inflammatory responses, especially if they were consumed prophylactically over extended periods of time. In these cases, questions should be considered about redirecting an immunological state of health. For example, if healthy individuals consume anti-inflammatory probiotic cultures regularly, might they become more susceptible to infectious pathogens?
M.V.K. As discussed above, it is not the prebiotic or probiotic alone that is essential for eliciting immunomodulatory effects. Equally or even more important are host-dependent factors, such as the genetic background of the individual, the composition of their specific gut microbiota, their diet and potentially other lifestyle factors. Although an understanding of the role of the microbiota on the epidermis, in the respiratory tract and in the gut is rapidly emerging, our insight into the complex interplay of the microbiota with its host is still limited, and the gastrointestinal tract remains a 'black box'. Hypothetically, a healthy subject might be colonized by a well-balanced composition of bacterial commensals, and supplementation with prebiotics or probiotics might enhance the risk of adverse outcomes instead of promoting beneficial health effects. It remains to be fully determined how specific prebiotics and probiotics might interact with the immune system. Moreover, further clinical trials are needed to identify susceptible subgroups that might benefit from prebiotics or probiotics34 and to carefully evaluate potential side effects.
M.K. Remarkably, the nutrigenomics studies discussed above5,6 revealed large differences between the mucosal transcriptome signatures from the individual participants, which appeared to be stable over time. Notably, the inter-individual differences in the signatures were approximately 10- to 100-fold greater than the differences elicited by probiotic consumption in an individual4,6, implying that mucosal homeostasis can be achieved via multiple molecular compositions, which we recently termed “the molecular bandwidth of health”4. Despite this 'individuality' of the volunteers, probiotic consumption elicited conserved and biologically coherent responses in all participants, including the transcriptional modulation of several stably expressed immune regulatory networks4,6. However, one can question whether the physiological consequences of probiotic consumption would be comparable in terms of disease-risk reduction in all individuals, as these consequences may depend strongly on the baseline molecular make-up of the individual. Differences in molecular make-up may explain so-called non-responders, who are frequently reported in probiotic intervention studies and other dietary intervention trials. Such baseline variation also implies that consumption of a specific probiotic strain with a defined immunomodulatory impact could be more effective in specific subpopulations of individuals with a particular immune phenotype, suggesting that improved prebiotic and probiotic efficacy may be achieved through more personalized or subpopulation-targeted approaches.
The requirement for personalization in pharmaceutical applications has been long recognized and has accelerated the development of advanced molecular diagnostics to improve personalized medicine approaches in the treatment of diseases35. Analogously, an improved understanding of the (molecular) individuality of humans, and how this affects the beneficial impact of prebiotics and probiotics on health, could significantly strengthen the scientific evidence to support the health claims associated with these products. Combined with deciphering the molecular mechanisms involved in the effects of prebiotic and probiotic consumption, these approaches can provide novel avenues for molecular science-based applications of probiotics in specific subpopulations of individuals.
Can prebiotics and/or probiotics be used to treat inflammatory diseases?
M.V.K. Apparently, the interaction of enteric bacteria and the intestinal epithelial mucosal immune system plays a crucial part in the development of IBD. However, there are insufficient data to recommend the routine use of prebiotics or probiotics for either the induction or maintenance of the remission of ulcerative colitis or Crohn's disease. Several Cochrane Reviews concluded that, although there are some promising results, there is a lack of well-designed randomized controlled clinical trials in this area, and further research is needed36,37.
There are indications of the efficacy of certain probiotics to reduce the risk of severe necrotizing enterocolitis (NEC) and mortality in preterm infants with birthweights above 1,000 g. However, data regarding the effect of probiotics on infants with extremely low birthweight are lacking. Furthermore, the potential for an increased risk of nosocomial sepsis in preterm infants given probiotics needs particular attention and careful evaluation. Therefore, insufficient evidence exists to recommend the routine use of probiotics for NEC38.
Based on the evidence emerging from clinical trials, probiotics or prebiotics cannot be used to treat — or prevent — any allergic disease. To date, 15 clinical trials29,34,39,40,41,42,43,44,45,46,47,48,49,50,51 targeting the primary prevention of allergy have been published and, consistently, none of these trials showed any effect on allergic sensitization, allergic rhinitis or bronchial asthma. There are also some conflicting data on the prevention of atopic eczema, but these trials vary considerably in terms of the intervention strategy and duration, end-point definition, follow-up period and selection criteria of the study population. Recently, Kuitunen et al. studied the effects of a mixture of different prebiotics and probiotics in 925 neonatal infants34. The percentage of atopic diseases was comparable between the prebiotic and probiotic group and the placebo group after 2 and 5 years. However, a post-hoc subgroup analysis revealed that the children who were delivered via caesarian section and received the prebiotics and probiotics had fewer IgE-associated diseases (24.3%) compared with the placebo group (40.5%) at the age of 5 years. It is tempting to speculate that children who were delivered via caesarian section might particularly benefit from probiotics, but data from prospectively designed studies are necessary to confirm this hypothesis.
In summary, the treatment of all inflammatory conditions using prebiotics and/or probiotics is hampered by a lack of convincing clinical trials with reproducible results. Although the concept is reasonable, it is unclear whether probiotics can progress from a promise to a reality for any clinical therapeutic or preventive approach.
T.R.K. IBDs such as Crohn's disease and ulcerative colitis have been a popular target for probiotic interventions. Moreover, inflammation also has been implicated in promoting polyposis and colon cancer52,53,54.
It is believed that both the commensal microbiota and probiotic microorganisms can exert protective effects by restoring microbial balance, enhancing epithelial barrier integrity and function, and reducing immune responses and inflammation55. However, human clinical research studies on the effect of probiotics on IBD are rare and the outcomes often ambiguous56. Therefore, researchers have recently focused on mouse models to investigate mechanisms by which probiotic microorganisms can modulate inflammation and either prevent or treat IBDs55. Administration of L. plantarum17 and L. rhamnosus GG57 with mutations that altered the D-alanine display on lipoteichoic acid, and therefore the cell surface charge, resulted in reduced colitis symptoms in mice. Furthermore, Mohamadzadeh et al.18 constructed a deletion mutant of phosphoglycerol transferase that completely removes lipoteichoic acid from the surface of L. acidophilus. This lipoteichoic acid-deficient probiotic induced anti-inflammatory cytokine profiles in DCs and, when delivered orally, prophylactically prevented colitis development in mice or alleviated colitis symptoms following disease induction. Moreover, owing to its anti-inflammatory properties, the lipoteichoic acid-deficient L. acidophilus was evaluated in a colon cancer mouse model and was shown to suppress the innate and adaptive pathogenic immune responses to protect against colonic polyposis58.
Indications from these studies are that alterations of the cell surface components of lactobacilli can alter the immunoregulatory responses of DCs and of the intestinal mucosa. The use of genetically modified lactobacilli that downregulate inflammatory responses could, in the future, be one important weapon in abating IBD and colon cancer73. In this regard, lactobacilli are considered 'generally recognized as safe' (GRAS) and have been consumed orally by humans for centuries. Directed modification of cell surface components for immunomodulation is now possible with genetic methods that make clean deletions (as with lipoteichoic acid in L. acidophilus) such that no foreign or recombinant DNA remains in the derived bacterium. As such, the continued GRAS status of such strains should be considered.
M.R. This is a very important question, particularly in light of the limited clinical benefit observed in several trials using probiotics in IBD36. In some gastrointestinal diseases, probiotics have proven to be beneficial, such as in antibiotic-associated diarrhoea, pouchitis, irritable bowel syndrome, Helicobacter pylori infection, Clostridium difficile disease and infectious diarrhoea59. In other diseases, such as traveller's diarrhoea and NEC, there was no observed effect59. However, the effect may be dependent on the strain of probiotic used in the trial. For instance, whereas several strains have proven beneficial in irritable bowel syndrome59, administration of L. plantarum MF1298 has been shown to have unfavourable effects60. If L. plantarum MF1298 has a pro-inflammatory function similar to that of L. plantarum v299, it is not surprising that the symptoms worsened. Indeed, L. plantarum v299 has been shown to cause destruction of healthy tissue8.
Interestingly, probiotics have been shown to have beneficial effects in pro-inflammatory diseases that are not located in the gastrointestinal tract, such as infant atopic dermatitis61, mastitis62 and possibly rheumatoid arthritis63. Also, prebiotics have been shown to have beneficial effects in some gastrointestinal disorders. For instance, oligofructose increased the numbers of faecal bifidobacteria and had a beneficial effect against relapse of C. difficile-associated diarrhoea64. A galactooligosaccharide mixture generated by a β-galactosidase from Bifidobacterium bifidum also increased the number of bifidobacterium species in healthy individuals65. Similarly, the prebiotic trans-galactooligosaccharide increased the representation of bifidobacteria in the stools of patients with irritable bowel syndrome, and this coincided with an alleviation of symptoms in a relatively small number of patients (three groups of 14–16 patients)66. Thus, although promising, more clinical studies are needed to confirm the beneficial activities of prebiotics in gastrointestinal disorders.
I do not think it is advisable to use probiotics during acute inflammation. Indeed, patients with acute pancreatitis experienced increased mortality after administration of a combination of three probiotics67. In an ex vivo organ culture model of inflamed intestines from patients with IBD, we also found that probiotics were inducing tissue destruction8 and, when administered before the induction of colitis in mice, two out of the three Lactobacillus strains assessed were shown to induce detrimental responses33.
By contrast, postbiotics may be a 'safe' alternative and may be preferable during the acute phase of inflammation because they can reduce an ongoing inflammatory response8. As mentioned above, several postbiotics have been identified as having protective effects in the gut and anti-inflammatory activities. In my opinion, by using defined postbiotics one may increase specificity and reduce undesired effects of probiotics, which have a large number of microorganism-associated molecular patterns that may precipitate the inflammatory response.
M.K. The question arises as to whether probiotics may be employed in therapeutic applications. For example, can probiotics repress inflammatory responses in individuals with mild or severe intestinal inflammation? Many of these diseases are associated with microbiota dysbiosis, suggesting that they may be affected by microbiota modulation. However, although the microbiota has a profound influence on the immune system1, these microorganism–host interactions are bidirectional, and the mucosal immune system can influence the composition and pro-inflammatory potential of the microbiota. For example, dysregulation of immune functions has been correlated with increased relative abundances of specific microbial groups in the microbiota that are associated with pro-inflammatory diseases68. Prebiotics and probiotics may help to restore normal microbiota communities in the intestine3 or may stimulate specific mucosal immune functions4, thereby contributing to the treatment of these diseases69. For example, mild inflammation in the intestinal mucosa may be amenable to prebiotic and probiotic treatment, and it has been suggested that probiotics could improve the inflammatory immune status in the elderly70. More severe intestinal inflammation, as is observed in patients with IBD, is associated with a loss of tolerance to the endogenous microbiota. This may suggest that tolerance induction by prebiotic or probiotic consumption may contribute to the treatment of these diseases, and some beneficial effects of probiotic consumption in IBD have been reported (for a review, see Ref. 69). However, tolerance induction by prebiotics or probiotics is unlikely to overrule host predisposition to diseases such as IBD, which is associated with several genetic factors and severe dysregulation of mucosal immune responses.
In addition, the most commonly applied probiotic genera — that is, the lactobacilli and bifidobacteria — may not be the most effective for the treatment of diseases. Indeed, the association of certain diseases with microbiota dysbiosis may offer alternative possibilities for probiotic therapy. An intriguing example is provided by the association of increased relative abundances of F. prausnitzii with extended remission periods in patients with Crohn's disease. F. prausnitzii was subsequently shown to elicit strong anti-inflammatory responses, suggesting that counterbalancing the dysbiosis by supplementing F. prausnitzii as a probiotic may benefit patients with Crohn's disease13. Analogously, the involvement of representatives of spore-forming Clostridium clusters IV and XIV in the induction of tolerant (TReg cell) responses in the colon may indicate their therapeutic potential in the treatment of diseases that are associated with loss of tolerance71.
All together, there may be considerable potential for the application of probiotics in the treatment of diseases that involve mild or severe mucosal inflammation in the intestine. However, it seems unlikely that the current repertoire of probiotic species is most suitable for such applications, and substantial therapeutic potential may be discovered in dietary management of the intestinal microbiota, including the development of probiotic therapies with 'novel' microbiota members.
References
Garrett, W. S., Gordon, J. I. & Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell 140, 859–870 (2010).
Zoetendal, E. G. et al. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 6, 1415–1426 (2012).
Reid, G. et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nature Rev. Microbiol. 9, 27–38 (2011).
Bron, P. A., van Baarlen, P. & Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nature Rev. Microbiol. 10, 66–78 (2011).
van Baarlen, P. et al. Differential NF-κB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl Acad. Sci. USA 106, 2371–2376 (2009).
van Baarlen, P. et al. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4562–4569 (2011).
Foligne, B. et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 13, 236–243 (2007).
Tsilingiri, K. et al. Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut 61, 1007–1015 (2012).
Fanning, S. et al. Bifidobacterial surface-exopolysaccharide facilitates commensal–host interaction through immune modulation and pathogen protection. Proc. Natl Acad. Sci. USA 109, 2108–2113 (2012).
Yan, F. et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Invest. 121, 2242–2253 (2011).
von Schillde, M. A. et al. Lactocepin secreted by lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 11, 387–396 (2012).
Segawa, S. et al. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin–p38 MAPK pathway. PLoS ONE 6, e23278 (2011).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Link-Amster, H., Rochat, F., Saudan, K. Y., Mignot, O. & Aeschlimann, J. M. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol. Med. Microbiol. 10, 55–63 (1994).
Christensen, H. R., Frøkiaer, H. & Pestka, J. J. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168, 171–178 (2002).
Konstantinov, S. R. et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl Acad. Sci. USA 105, 19474–19479 (2008).
Grangette, C. et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc. Natl Acad. Sci. USA 102, 10321–10326 (2005).
Mohamadzadeh, M. et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4623–4630 (2011).
Macho Fernandez, E. et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 60, 1050–1059 (2011).
Lebeer, S., Claes, I. J., Verhoeven, T. L., Vanderleyden, J. & De Keersmaecker, S. C. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb. Biotechnol. 4, 368–374 (2011).
Lebeer, S. et al. The major secreted protein Msp1/p75 is O glycosylated in Lactobacillus rhamnosus GG. Microb. Cell Fact. 11, 15 (2012).
Lebeer, S. et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 78, 185–193 (2012).
Lebeer, S., Claes, I. J. & Vanderleyden, J. Anti-inflammatory potential of probiotics: lipoteichoic acid makes a difference. Trends Microbiol. 20, 5–10 (2012).
Lebeer, S., Vanderleyden, J. & De Keersmaecker, S. C. J. Host interactions of probiotic bacterial surface molecules: comparisons with commensals and pathogens. Nature Rev. Microbiol. 8, 171–184 (2010).
Mazmanian, S. K. & Kasper, D. L. The love–hate relationship between bacterial polysaccharides and the host immune system. Nature Rev. Immunol. 6, 849–858 (2006).
Campeotto, F. et al. A fermented formula in pre-term infants: clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 22, 1–10 (2011).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Kopp, M. V. et al. Lactobacillus GG has in vitro effects on enhanced IL-10 and IFN-γ release of mononuclear cells but no in vivo effects in supplemented mothers and their neonates. Clin. Exp. Allergy 38, 602–610 (2008).
Kopp, M. V. et al. A randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical or immunological effects of Lactobacillus GG supplementation. Pediatrics 121, e850–e856 (2008).
Konieczna, P. et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut 61, 354–366 (2012).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473, 174–180 (2011).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Mileti, E., Matteoli, G., Iliev, I. D., Rescigno, M. Comparison of the immunomodulatory properties of three probiotic strains of lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS ONE 4, e7056 (2009).
Kuitunen, M. et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 123, 335–341 (2009).
La Thangue, N. B. & Kerr, D. J. Predictive biomarkers: a paradigm shift towards personalized cancer medicine. Nature Rev. Clin. Oncol. 8, 587–596 (2011).
Naidoo, K., Gordon, M., Fagbemi, A. O., Thomas, A. G. & Akobeng, A. K. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2011, CD007443 (2011).
Butterworth, A. D., Thomas, A. G. & Akobeng, A. K. Probiotics for induction of remission in Crohn's disease. Cochrane Database Syst. Rev. 2008, CD006634 (2008).
Mihatsch, W. A. et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin. Nutr. 31, 6–15 (2012).
Kalliomaki, M. et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357, 1076–1079 (2001).
Taylor, A. L., Dunstan, J. A. & Prescott, S. L. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J. Allergy Clin. Immunol. 119, 184–191 (2007).
Abrahamsson, T. R. et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1174–1180 (2007).
Wickens, K. et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 122, 788–794 (2008).
Hurree, A. et al. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitizations: a double-blind, placebo controlled study. Clin. Exp. Allergy 38, 1342–1348 (2008).
Dotterud, C. K., Storro, O., Johnsen, R. & Oien, T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br. J. Dermatol. 163, 616–623 (2010).
Boyle, R. J. et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy 66, 509–516 (2011).
Niers, L. et al. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy 64, 1349–1358 (2009).
Soh, S. E. et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants – effects on eczema and atopic sensitization at the age of 1 year. Clin. Exp. Allergy 39, 571–578 (2009).
West, C. E., Hammarstrom, M. L. & Hernell, O. Probiotics during weaning reduce the incidence of eczema. Pediatr. Allergy Immunol. 20, 430–437 (2009).
Kim, J. Y. et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr. Allergy Immunol. 21, e386–e393 (2009).
Gruber, C. et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low atopy risk infants. J. Allergy Clin. Immunol. 126, 791–797 (2010).
Moro, G. et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 91, 814–819 (2006).
Gounaris, E. et al. T regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 69, 5490–5497 (2009).
Blatner, N. R. et al. In colorectal cancer mast cells contribute to systemic regulatory T cell dysfunction. Proc. Natl Acad. Sci. USA 107, 6430–6435 (2010).
Khazaie, K. et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 30, 45–60 (2011).
Claes, I. J. J., De Keersmaecker, S. C. J., Vanderleyden, J. & Lebeer, S. Lessons from probiotic–host interaction studies in murine models of experimental colitis. Mol. Nutr. Food Res. 55, 1441–1453 (2011).
Sartor, R. B. Efficacy of probiotics for the management of inflammatory bowel disease. Gastroenterol. Hepatol. 7, 606–608 (2011).
Claes, I. J. et al. Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin. Exp. Immunol. 162, 306–314 (2010).
Khazaie, K. et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl Acad. Sci. USA 109, 10462–10467 (2012).
Ritchie, M. L. & Romanuk, T. N. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE 7, e34938 (2012).
Ligaarden, S. C., Axelsson, L., Naterstad, K., Lydersen, S. & Farup, P. G. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: a randomised controlled trial. BMC Gastroenterol. 10, 16 (2010).
Pelucchi, C. et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 23, 402–414 (2012).
Arroyo, R. et al. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of lactobacilli isolated from breast milk. Clin. Infect. Dis. 50, 1551–1558 (2010).
Pineda Mde, L. et al. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med. Sci. Monit. 17, CR347–CR354 (2011).
Lewis, S., Burmeister, S. & Brazier, J. Effect of the prebiotic oligofructose on relapse of Clostridium difficile-associated diarrhea: a randomized, controlled study. Clin. Gastroenterol. Hepatol. 3, 442–448 (2005).
Depeint, F., Tzortzis, G., Vulevic, J., I'anson, K. & Gibson, G. R. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: a randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr. 87, 785–791 (2008).
Silk, D. B., Davis, A., Vulevic, J., Tzortzis, G. & Gibson, G. R. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 29, 508–518 (2009).
Besselink, M. G. et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 371, 651–659 (2008).
DuPont, A. W. & DuPont, H. L. The intestinal microbiota and chronic disorders of the gut. Nature Rev. Gastroenterol. Hepatol. 8, 523–531 (2011).
Gareau, M. G., Sherman, P. M. & Walker, W. A. Probiotics and the gut microbiota in intestinal health and disease. Nature Rev. Gastroenterol. Hepatol. 7, 503–514 (2010).
Guigoz, Y., Doré, J. & Schiffrin, E. J. The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care 11, 13–20 (2008).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Kleerebezem, M. et al. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 34, 199–230 (2010).
Khazaie, K. et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl Acad. Sci. USA 109, 10462–10467 (2012).
Acknowledgements
M.R. is supported by the European Research Council, the European Commission (FP7: IBDase, MetaHIT), the Italian Ministry of Health, the Association for International Cancer Research and the Italian Association for Cancer Research.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
T.R.K. has received grants from Danisco USA & DuPont Nutrition & Health, and Dairy Research, Inc., for research on the functional genomics of probiotic lactobacilli.
M.K. declares no competing financial interests.
M.V.K. has received research funds for clinical trials from Infectopharm (Heppenheim, Germany) and Novartis Pharma (Nuernberg, Germany). M.V.K. has consultant arrangements with Infectopharm (Heppenheim, Germany), Novartis Pharma (Nuernberg, Germany), Nutricia (Erlangen, Germany) and Bencard (Muenchen, Germany).
M.R. declares no competing financial interests.
Related links
Glossary
- Epidermal growth factor receptor
-
(EGFR). A cell-surface receptor that binds a family of growth factors that includes EGF and transforming growth factor-β (TGFβ).
- Inflammatory bowel disease
-
(IBD). A group of conditions, of unknown aetiology, in which the intestinal mucosa is chronically inflamed. Includes Crohn's disease and ulcerative colitis.
- Lipoteichoic acid
-
A major constituent of the cell wall of Gram-positive bacteria. The structure of lipoteichoic acid varies between the different species of Gram-positive bacteria and may contain long chains of ribitol or glycerol phosphate. It is anchored to the cell membrane via a glyceride and can stimulate specific immune responses.
- Necrotizing enterocolitis
-
(NEC). A gastrointestinal disease predominantly affecting premature infants with low birth-weight. NEC involves infection and inflammation that causes destruction of the intestine. Although the pathophysiology of NEC is not yet completely defined, increasing evidence indicates that immaturity of intestinal innate immune function in the premature gut is a major factor.
- Prebiotics
-
Non-digestible food ingredients that stimulate the growth and/or activity of bacteria in the digestive system.
- Probiotics
-
Live microorganisms that when administered in adequate amounts confer a health benefit on the host.
- The molecular bandwidth of health
-
The differences in the 'stable' baseline molecular makeup of mucosal tissue in the intestine of healthy human individuals.
Rights and permissions
About this article
Cite this article
Klaenhammer, T., Kleerebezem, M., Kopp, M. et al. The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol 12, 728–734 (2012). https://doi.org/10.1038/nri3312
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3312
This article is cited by
-
Effect of prebiotics, probiotics, synbiotics on depression: results from a meta-analysis
BMC Psychiatry (2023)
-
The panda-derived Lactiplantibacillus plantarum BSG201683 improves LPS-induced intestinal inflammation and epithelial barrier disruption in vitro
BMC Microbiology (2023)
-
Strain-specific alterations in gut microbiome and host immune responses elicited by tolerogenic Bifidobacterium pseudolongum
Scientific Reports (2023)
-
Protective Effects of the Postbiotic Lactobacillus plantarum MD35 on Bone Loss in an Ovariectomized Mice Model
Probiotics and Antimicrobial Proteins (2023)
-
Probiotic potential of Tetragenococcus halophilus EFEL7002 isolated from Korean soy Meju
BMC Microbiology (2022)