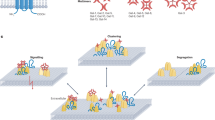
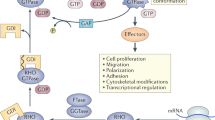
Key Points
-
Scaffold proteins have an important role in regulating immune-cell signalling. This Review provides an overview of the numerous functions that have been attributed to scaffold proteins, and discusses various cytoplasmic scaffold proteins that are important in immune cells.
-
Although little is known about the exact role of scaffold proteins, mathematical modelling and engineered scaffold proteins have greatly enhanced our knowledge of their function.
-
Initial studies of scaffold proteins indicate that they are important for spatial localization and amplification of signal transduction.
-
Scaffolds can generate complex behaviours that include transient or sustained signalling, and oscillatory signalling, as well as provide positive and negative feedback.
-
In immune cells, scaffold proteins have an important role in the regulation of mitogen-activated protein kinase activation, calcium signalling, signalling downstream of innate immune receptors and cell polarity.
-
The function and regulation of scaffold proteins is complex and much remains to be defined. It is probable that new tools, in addition to classic biochemical approaches, will be required to elucidate these functions.
Abstract
Over the past 20 years great progress has been made in defining most of the key signalling pathways that functionally regulate immune cells. Recently, it has become clear that scaffold proteins have a crucial role in regulating many of these signalling cascades. By binding two or more components of a signalling pathway, scaffold proteins can help to localize signalling molecules to a specific part of the cell or to enhance the efficacy of a signalling pathway. Scaffold proteins can also affect the thresholds and the dynamics of signalling reactions by coordinating positive and negative feedback signals. In this Review, we focus on recent progress in the understanding of the function of scaffold proteins in immune cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cooper, J. A., Bowen-Pope, D. F., Raines, E., Ross, R. & Hunter, T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell 31, 263–273 (1982).
Wang, Y., Pennock, S., Chen, X. & Wang, Z. Internalization of inactive EGF receptor into endosomes and the subsequent activation of endosome-associated EGF receptors. Epidermal growth factor. Sci. STKE 2002, PL17 (2002).
Wang, Y., Pennock, S. D., Chen, X., Kazlauskas, A. & Wang, Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J. Biol. Chem. 279, 8038–8046 (2004).
Kane, L. P., Lin, J. & Weiss, A. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12, 242–249 (2000).
Takeda, K., Kaisho, T. & Akira, S. Toll-like receptors. Annu. Rev. Immunol. 21, 335–376 (2003).
Aggarwal, B. B. Signalling pathways of the TNF superfamily: a double-edged sword. Nature Rev. Immunol. 3, 745–756 (2003).
Aguado, E., Martinez-Florensa, M. & Aparicio, P. Activation of T lymphocytes and the role of the adapter LAT. Transpl. Immunol. 17, 23–26 (2006).
Wu, J. N. & Koretzky, G. A. The SLP-76 family of adapter proteins. Semin. Immunol. 16, 379–393 (2004).
Burack, W. R., Cheng, A. M. & Shaw, A. S. Scaffolds, adaptors and linkers of TCR signaling: theory and practice. Curr. Opin. Immunol. 14, 312–316 (2002).
Nakanishi, H. et al. Neurabin: a novel neural tissue-specific actin filament-binding protein involved in neurite formation. J. Cell Biol. 139, 951–961 (1997).
Levchenko, A., Bruck, J. & Sternberg, P. W. Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc. Natl Acad. Sci. USA 97, 5818–5823 (2000).
Ferrell, J. E. Jr. What do scaffold proteins really do? Sci. STKE 2000, PE1 (2000).
Burack, W. R. & Shaw, A. S. Signal transduction: hanging on a scaffold. Curr. Opin. Cell Biol. 12, 211–216 (2000).
Wong, W. & Scott, J. D. AKAP signalling complexes: focal points in space and time. Nature Rev. Mol. Cell Biol. 5, 959–970 (2004).
Williams, R. O. Cutting Edge: A-kinase anchor proteins are involved in maintaining resting T cells in an inactive state. J. Immunol. 168, 5392–5396 (2002).
Locasale, J. W., Shaw, A. S. & Chakraborty, A. K. Scaffold proteins confer diverse regulatory properties to protein kinase cascades. Proc. Natl Acad. Sci. USA 104, 13307–13312 (2007). This study uses mathematical modelling to examine possible functions of scaffold proteins and identifies important variables that might alter these functions.
Pincet, F. Membrane recruitment of scaffold proteins drives specific signaling. PLoS ONE 2, e977 (2007).
Uhlik, M. T., Abell, A. N., Cuevas, B. D., Nakamura, K. & Johnson, G. L. Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem. Cell Biol. 82, 658–663 (2004).
Bashor, C. J., Helman, N. C., Yan, S. & Lim, W. A. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science 319, 1539–1543 (2008). Using engineered scaffold proteins to recruit pathway modulators to a signalling cascade, this study identifies a range of signalling behaviours that can be induced by scaffold proteins.
Park, S. H., Zarrinpar, A. & Lim, W. A. Rewiring MAP kinase pathways using alternative scaffold assembly mechanisms. Science 299, 1061–1064 (2003).
Dueber, J. E., Mirsky, E. A. & Lim, W. A. Engineering synthetic signaling proteins with ultrasensitive input/output control. Nature Biotechnol. 25, 660–662 (2007).
Lewis, R. S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 (2001).
Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nature Rev. Mol. Cell Biol. 6, 827–837 (2005).
Elion, E. A. The Ste5p scaffold. J. Cell Sci. 114, 3967–3978 (2001).
Claperon, A. & Therrien, M. KSR and CNK: two scaffolds regulating RAS-mediated RAF activation. Oncogene 26, 3143–3158 (2007).
Therrien, M. et al. KSR, a novel protein kinase required for RAS signal transduction. Cell 83, 879–888 (1995).
Kornfeld, K., Hom, D. B. & Horvitz, H. R. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell 83, 903–913 (1995).
Sundaram, M. & Han, M. The C. elegans ksr-1 gene encodes a novel Raf-related kinase involved in Ras-mediated signal transduction. Cell 83, 889–901 (1995).
Cacace, A. M. et al. Identification of constitutive and Ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol. Cell. Biol. 19, 229–240 (1999).
Muller, J., Ory, S., Copeland, T., Piwnica-Worms, H. & Morrison, D. K. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8, 983–993 (2001).
Douziech, M., Sahmi, M., Laberge, G. & Therrien, M. A KSR/CNK complex mediated by HYP, a novel SAM domain-containing protein, regulates RAS-dependent RAF activation in Drosophila. Genes Dev. 20, 807–819 (2006).
Therrien, M., Wong, A. M. & Rubin, G. M. CNK, a RAF-binding multidomain protein required for RAS signaling. Cell 95, 343–353 (1998).
Boudeau, J., Miranda-Saavedra, D., Barton, G. J. & Alessi, D. R. Emerging roles of pseudokinases. Trends Cell Biol. 16, 443–452 (2006).
Huang, C. L., Cha, S. K., Wang, H. R., Xie, J. & Cobb, M. H. WNKs: protein kinases with a unique kinase domain. Exp. Mol. Med. 39, 565–573 (2007).
Mukherjee, K. et al. CASK functions as a Mg2+– independent neurexin kinase. Cell 133, 328–339 (2008).
Ohmachi, M. et al. C. elegans ksr-1 and ksr-2 have both unique and redundant functions and are required for MPK-1 ERK phosphorylation. Curr. Biol. 12, 427–433 (2002).
Channavajhala, P. L. et al. Identification of a novel human kinase supporter of Ras (hKSR-2) that functions as a negative regulator of Cot (Tpl2) signaling. J. Biol. Chem. 278, 47089–47097 (2003).
Nguyen, A. et al. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol. Cell. Biol. 22, 3035–3045 (2002).
Fischer, A. M., Katayama, C. D., Pages, G., Pouyssegur, J. & Hedrick, S. M. The role of Erk1 and Erk2 in multiple stages of T cell development. Immunity 23, 431–443 (2005).
Fusello, A. M. et al. The MAPK scaffold kinase suppressor of Ras is involved in ERK activation by stress and proinflammatory cytokines and induction of arthritis. J. Immunol. 177, 6152–6158 (2006).
Rincon, M. & Pedraza-Alva, G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol. Rev. 192, 131–142 (2003).
Dong, C., Davis, R. J. & Flavell, R. A. MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Yasuda, J., Whitmarsh, A. J., Cavanagh, J., Sharma, M. & Davis, R. J. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19, 7245–7254 (1999).
Willoughby, E. A., Perkins, G. R., Collins, M. K. & Whitmarsh, A. J. The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J. Biol. Chem. 278, 10731–10736 (2003).
Verhey, K. J. et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 152, 959–970 (2001).
Gallagher, E. et al. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nature Immunol. 8, 57–63 (2007).
Su, Y. C., Han, J., Xu, S., Cobb, M. & Skolnik, E. Y. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J. 16, 1279–1290 (1997).
Matsuzawa, A. et al. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science 321, 663–668 (2008). This paper proposes a two-step mechanism for signalling by CD40 in which a CD40-associated signalling complex that is scaffolded by MEKK1 must be released from the receptor into the cytoplasm for signal propagation.
Blonska, M. et al. The CARMA1–Bcl10 signaling complex selectively regulates JNK2 kinase in the T cell receptor-signaling pathway. Immunity 26, 55–66 (2007).
Lin, X. & Wang, D. The roles of CARMA1, Bcl10, and MALT1 in antigen receptor signaling. Semin. Immunol. 16, 429–435 (2004).
Feske, S. Calcium signalling in lymphocyte activation and disease. Nature Rev. Immunol. 7, 690–702 (2007).
Haase, H. et al. Ahnak is critical for cardiac Ca(v)1.2 calcium channel function and its β-adrenergic regulation. FASEB J. 19, 1969–1977 (2005).
Lee, I. H. et al. Ahnak protein activates protein kinase C (PKC) through dissociation of the PKC-protein phosphatase 2A complex. J. Biol. Chem. 283, 6312–6320 (2008).
Lee, I. H. et al. AHNAK-mediated activation of phospholipase C-γ1 through protein kinase C. J. Biol. Chem. 279, 26645–26653 (2004).
Matza, D. et al. A scaffold protein, AHNAK1, is required for calcium signaling during T cell activation. Immunity 28, 64–74 (2008). This study establishes a role for AHNAK1 in calcium signalling in T cells.
Sekiya, F., Bae, Y. S., Jhon, D. Y., Hwang, S. C. & Rhee, S. G. AHNAK, a protein that binds and activates phospholipase C-γ1 in the presence of arachidonic acid. J. Biol. Chem. 274, 13900–13907 (1999).
Shiraishi-Yamaguchi, Y. & Furuichi, T. The Homer family proteins. Genome Biol. 8, 206 (2007).
Thomas, U. Modulation of synaptic signalling complexes by Homer proteins. J. Neurochem. 81, 407–413 (2002).
Xiao, B., Tu, J. C. & Worley, P. F. Homer: a link between neural activity and glutamate receptor function. Curr. Opin. Neurobiol. 10, 370–374 (2000).
Stiber, J. A. et al. Homer modulates NFAT-dependent signaling during muscle differentiation. Dev. Biol. 287, 213–224 (2005).
Huang, G. N. et al. NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science 319, 476–481 (2008). This is the first study to show a role for HOMER proteins in calcium signalling in T cells.
Ninomiya-Tsuji, J. et al. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252–256 (1999).
Schauvliege, R., Janssens, S. & Beyaert, R. Pellino proteins: novel players in TLR and IL-1R signalling. J. Cell. Mol. Med. 11, 453–461 (2007).
Grosshans, J., Schnorrer, F. & Nusslein-Volhard, C. Oligomerisation of Tube and Pelle leads to nuclear localisation of dorsal. Mech. Dev. 81, 127–138 (1999).
Jiang, Z. et al. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)–IRAK–tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J. Biol. Chem. 278, 10952–10956 (2003).
Yu, K. Y. et al. Cutting Edge: mouse pellino-2 modulates IL-1 and lipopolysaccharide signaling. J. Immunol. 169, 4075–4078 (2002).
Jensen, L. E. & Whitehead, A. S. Pellino3, a novel member of the Pellino protein family, promotes activation of c-Jun and Elk-1 and may act as a scaffolding protein. J. Immunol. 171, 1500–1506 (2003).
Liu, Y. et al. BCL10 mediates lipopolysaccharide/toll-like receptor-4 signaling through interaction with Pellino2. J. Biol. Chem. 279, 37436–37444 (2004).
Ting, J. P., Willingham, S. B. & Bergstralh, D. T. NLRs at the intersection of cell death and immunity. Nature Rev. Immunol. 8, 372–379 (2008).
Martinon, F., Gaide, O., Petrilli, V., Mayor, A. & Tschopp, J. NALP inflammasomes: a central role in innate immunity. Semin. Immunopathol. 29, 213–229 (2007).
Petrilli, V., Dostert, C., Muruve, D. A. & Tschopp, J. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19, 615–622 (2007).
Aganna, E. et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452 (2002).
Jin, Y. et al. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 356, 1216–1225 (2007).
Eisenbarth, S. C., Colegio, O. R., O'Connor, W., Sutterwala, F. S. & Flavell, R. A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008).
Bruey, J. M. et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell 129, 45–56 (2007). This interesting study connects the NLRP1 inflammasome with the cell-death pathway and shows that anti-apoptotic molecules can inhibit the function of NLRP1.
Mayor, A., Martinon, F., De Smedt, T., Petrilli, V. & Tschopp, J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nature Immunol. 8, 497–503 (2007).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 (2007).
Hall, Z. W. & Sanes, J. R. Synaptic structure and development: the neuromuscular junction. Cell 72, S99–S121 (1993).
Cemerski, S. & Shaw, A. Immune synapses in T-cell activation. Curr. Opin. Immunol. 18, 298–304 (2006).
Kennedy, M. B. Signal-processing machines at the postsynaptic density. Science 290, 750–754 (2000).
Gonzalez-Mariscal, L., Betanzos, A. & Avila-Flores, A. MAGUK proteins: structure and role in the tight junction. Semin. Cell Dev. Biol. 11, 315–324 (2000).
Kim, E. & Sheng, M. PDZ domain proteins of synapses. Nature Rev. Neurosci. 5, 771–781 (2004).
Montgomery, J. M., Zamorano, P. L. & Garner, C. C. MAGUKs in synapse assembly and function: an emerging view. Cell. Mol. Life Sci. 61, 911–929 (2004).
Stephenson, L. M. et al. DLGH1 is a negative regulator of T-lymphocyte proliferation. Mol. Cell. Biol. 27, 7574–7581 (2007).
Xavier, R. et al. Discs large (Dlg1) complexes in lymphocyte activation. J. Cell Biol. 166, 173–178 (2004).
Hanada, T., Lin, L., Chandy, K. G., Oh, S. S. & Chishti, A. H. Human homologue of the Drosophila discs large tumor suppressor binds to p56lck tyrosine kinase and Shaker type Kv1.3 potassium channel in T lymphocytes. J. Biol. Chem. 272, 26899–26904 (1997).
Round, J. L. et al. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J. Exp. Med. 201, 419–430 (2005).
Round, J. L. et al. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-κB transcription factors. Nature Immunol. 8, 154–161 (2007).
Ashwell, J. D. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nature Rev. Immunol. 6, 532–540 (2006).
Yang, T. T., Xiong, Q., Enslen, H., Davis, R. J. & Chow, C. W. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol. Cell. Biol. 22, 3892–3904 (2002).
Gomez del Arco, P., Martinez-Martinez, S., Maldonado, J. L., Ortega-Perez, I. & Redondo, J. M. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem. 275, 13872–13878 (2000).
Wu, C. C., Hsu, S. C., Shih, H. M. & Lai, M. Z. Nuclear factor of activated T cells c is a target of p38 mitogen-activated protein kinase in T cells. Mol. Cell. Biol. 23, 6442–6454 (2003).
Cemerski, S. et al. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity 26, 345–355 (2007).
Krummel, M. F. & Macara, I. Maintenance and modulation of T cell polarity. Nature Immunol. 7, 1143–1149 (2006).
Ludford-Menting, M. J. et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity 22, 737–748 (2005).
Allen, P. B., Ouimet, C. C. & Greengard, P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc. Natl Acad. Sci. USA 94, 9956–9961 (1997).
Zito, K., Knott, G., Shepherd, G. M., Shenolikar, S. & Svoboda, K. Induction of spine growth and synapse formation by regulation of the spine actin cytoskeleton. Neuron 44, 321–334 (2004).
Sarrouilhe, D., di Tommaso, A., Metaye, T. & Ladeveze, V. Spinophilin: from partners to functions. Biochimie 88, 1099–1113 (2006).
Bloom, O. et al. Spinophilin participates in information transfer at immunological synapses. J. Cell Biol. 181, 203–211 (2008).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
FURTHER INFORMATION
Glossary
- Immunological synapse
-
A large junctional structure that is formed at the cell surface between a T cell that is interacting with an APC or a target cell, which consists of molecules that are required for adhesion and signalling. This structure is important for establishing T-cell adhesion and polarity, is influenced by the cytoskeleton and transduces highly controlled secretory signals, thereby allowing the directed release of cytokines or lytic granules towards the APC or target cell.
- 14-3-3 proteins
-
A family of conserved proteins that is present in all eukaryotic organisms and is involved in diverse cellular processes, such as apoptosis and stress, as well as intracellular signalling and cell-cycle regulation. 14-3-3 proteins function as scaffolds in protein interactions and can regulate protein localization and enzymatic activity. Approximately 100 binding partners for the 14-3-3 proteins have been reported.
- Caspase-recruitment domain
-
A domain that is found in certain initiator caspases (for example, mammalian caspase 9) and their adaptor proteins (for example, APAF1). This domain mediates protein–protein interactions.
- Nuclear export signal
-
A short amino-acid sequence of 5–6 hydrophobic residues that targets a protein for export from the cell nucleus to the cytoplasm.
- Leucine zipper
-
A common dimerization domain found in some proteins that are involved in regulating gene expression. Leucine zipper refers to the secondary structure of two parallel α-helices found in the protein.
- Effector memory T cell
-
A terminally differentiated T cell that lacks lymph-node-homing receptors but expresses receptors that enable it to home to inflamed tissues. Effector memory cells can exert immediate effector functions without the need for further differentiation.
- Inflammasome
-
A molecular complex of several proteins that, once assembled, cleaves pro-IL-1β, thereby producing active IL-1β.
- Vitiligo
-
A depigmenting disorder of the skin caused by the destruction of melanocytes, which produce cutaneous pigments.
- Short hairpin RNA
-
One of the two most common forms of short (∼21 base pairs) double-stranded RNAs that are used for gene silencing. The other form is small interfering RNA.
- Uropod
-
The posterior tail of migrating amoeboid cells. It is rich in filamentous actin, microtubules and cytoskeletal adaptor proteins (such as ezrin and moesin), as well as adhesion molecules (such as CD43 and CD44) and lipid rafts.
Rights and permissions
About this article
Cite this article
Shaw, A., Filbert, E. Scaffold proteins and immune-cell signalling. Nat Rev Immunol 9, 47–56 (2009). https://doi.org/10.1038/nri2473
Issue Date:
DOI: https://doi.org/10.1038/nri2473
This article is cited by
-
Mathematical model of the cell signaling pathway based on the extended Boolean network model with a stochastic process
BMC Bioinformatics (2022)
-
Discussing the final size and shape of the reconstructed tissues in tissue engineering
Journal of Artificial Organs (2022)
-
S-acylation-dependent membrane microdomain localization of the regulatory Kvβ2.1 subunit
Cellular and Molecular Life Sciences (2022)
-
Unifying principles of bifunctional, proximity-inducing small molecules
Nature Chemical Biology (2020)
-
ScaPD: a database for human scaffold proteins
BMC Bioinformatics (2017)