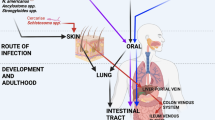
Key Points
-
In the last 20–30 years there has been a dramatic escalation in the prevalence of allergic diseases in industrialized countries. The aetiology of this rise is not known.
-
Previously, the hygiene hypothesis has proposed that the absence of or reduction in infectious diseases might be a factor for the increase in the prevalence of allergies in industrialized societies. However, this is only one aspect of the multifactorial changes in immune stimuli that have occurred in the past few decades.
-
In support of a potential role for parasitic helminths in the rise in allergies, an association between infection with helminth parasites and a lower allergic phenotype has been observed in human infections in societies in which helminths are prevalent. Mechanistically, a potential role for helminth-induced IL-10 expression has been implicated in this inverse association.
-
In experimental animal models, helminths can modulate allergic responses, including anaphylaxis, allergen-induced eosinophilia and compromised lung function. Potential immunological mechanisms postulated for these responses include regulatory T cells and the expression of the regulatory cytokine IL-10 from various cell sources.
-
We propose that helminths induce a modified pulmonary type 2 response that suppresses inflammation in the lungs. Helminths might have evolved this mechanism of suppression of lung inflammation to prevent death of the host that is due to pulmonary pathology as new infecting larvae migrate through the lungs.
-
Future research unifying key data from humans infected with helminths or experimental animal infections will identify common mechanisms of suppression of allergies in infection. Understanding these mechanisms will lead to new therapeutic strategies for the treatment of allergic diseases.
Abstract
There is no immunological mechanism to adequately explain the sudden epidemic in allergies noted in the last 30 years in developed countries. The reduction in the development of allergic disorders observed in individuals infected with parasitic helminths, however, supports a possible role for worms in suppressing allergies. Helminths regulate the immunity of the host to ensure a mutually beneficial environment for the survival of both the parasite and the host. This interplay between helminths and allergic responses raises fundamental questions in immunobiology. Harnessing current mechanistic studies for translational research into helminth infections and atopy might have potential for the identification of novel biomarkers, and even therapeutics, in allergic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Braman, S. S. The global burden of asthma. Chest 130 (Suppl. 1), 4–12 (2006).
Zollner, I. K. et al. No increase in the prevalence of asthma, allergies, and atopic sensitisation among children in Germany: 1992–2001. Thorax 60, 545–548 (2005).
Strachan, D. P. Hay fever, hygiene, and household size. BMJ 299, 1259–1260 (1989).
Wills-Karp, M., Santeliz, J. & Karp, C. L. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nature Rev. Immunol. 1, 69–75 (2001). This is a review of the immunologiocal basis of the hygiene hypothesis.
Umetsu, D. T., McIntire, J. J., Akbari, O., Macaubas, C. & DeKruyff, R. H. Asthma: an epidemic of dysregulated immunity. Nature Immunol. 3, 715–720 (2002).
Noverr, M. C. & Huffnagle, G. B. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 12, 562–568 (2004).
Mazmanian, S. K. & Kasper, D. L. The love–hate relationship between bacterial polysaccharides and the host immune system. Nature Rev. Immunol. 6, 849–858 (2006). This paper explores the potential role of bacteria in the hygiene hypothesis
Vercelli, D. Mechanisms of the hygiene hypothesis — molecular and otherwise. Curr. Opin. Immunol. 18, 733–737 (2006).
Weiss, S. T. Obesity: insight into the origins of asthma. Nature Immunol. 6, 537–539 (2005).
Waite, D. A., Eyles, E. F., Tonkin, S. L. & O'Donnell, T. V. Asthma prevalence in Tokelauan children in two environments. Clin. Allergy 10, 71–75 (1980).
Ege, M. J. et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J. Allergy Clin. Immunol. 117, 817–823 (2006).
Lane, J., Siebers, R., Pene, G., Howden-Chapman, P. & Crane, J. Tokelau: a unique low allergen environment at sea level. Clin. Exp. Allergy 35, 479–482 (2005).
Renz, H. et al. TH1/TH2 immune response profiles differ between atopic children in eastern and western Germany. J. Allergy Clin. Immunol. 109, 338–342 (2002).
Krause, T. et al. Frequency of atopy in the Arctic in 1987 and 1998. Lancet 360, 691–692 (2002).
Ober, C. & Hoffjan, S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 7, 95–100 (2006).
Romagnani, S. Regulation of the development of type 2 T-helper cells in allergy. Curr. Opin. Immunol. 6, 838–846 (1994).
Yazdanbakhsh, M., Kremsner, P. G. & van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 296, 490–494 (2002).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Dunne, D. W. & Cooke, A. A worm's eye view of the immune system: consequences for evolution of human autoimmune disease. Nature Rev. Immunol. 5, 420–426 (2005).
Maizels, R. M. & Yazdanbakhsh, M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Rev. Immunol. 3, 733–744 (2003).
Van Dellen, R. G. & Thompson, J. H. Jr. Absence of intestinal parasites in asthma. N. Engl. J. Med. 285, 146–148 (1971).
Stoll, N. R. This wormy world. 1947. J. Parasitol. 85, 392–396 (1999).
Chan, M. S. The global burden of intestinal nematode infections — fifty years on. Parasitol. Today 13, 438–443 (1997).
Lynch, N. R. et al. Allergic reactivity and socio-economic level in a tropical environment. Clin. Allergy 17, 199–207 (1987).
Scrivener, S. et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case–control study. Lancet 358, 1493–1499 (2001).
Nyan, O. A. et al. Atopy, intestinal helminth infection and total serum IgE in rural and urban adult Gambian communities. Clin. Exp. Allergy 31, 1672–1678 (2001).
Huang, S. L., Tsai, P. F. & Yeh, Y. F. Negative association of Enterobius infestation with asthma and rhinitis in primary school children in Taipei. Clin. Exp. Allergy 32, 1029–1032 (2002).
Cooper, P. J. et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J. Allergy Clin. Immunol. 111, 995–1000 (2003).
Lynch, N. R. et al. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J. Allergy Clin. Immunol. 92, 404–411 (1993).
Borkow, G. et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J. Clin. Invest. 106, 1053–1060 (2000).
van den Biggelaar, A. H. et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J. Infect. Dis. 189, 892–900 (2004).
Palmer, L. J. et al. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am. J. Respir. Crit. Care Med. 165, 1489–1493 (2002).
Joubert, J. R., van Schalkwyk, D. J. & Turner, K. J. Ascaris lumbricoides and the human immunogenic response: enhanced IgE-mediated reactivity to common inhaled allergens. S. Afr. Med. J. 57, 409–412 (1980).
Dold, S., Heinrich, J., Wichmann, H. E. & Wjst, M. Ascaris-specific IgE and allergic sensitization in a cohort of school children in the former East Germany. J. Allergy Clin. Immunol. 102, 414–420 (1998).
Cooper, P. J. et al. Effect of albendazole treatments on the prevalence of atopy in children living in communities endemic for geohelminth parasites: a cluster-randomised trial. Lancet 367, 1598–1603 (2006).
Leonardi-Bee, J., Pritchard, D. & Britton, J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am. J. Respir. Crit. Care Med. 174, 514–523 (2006). This is a comprehensive analysis of published studies on the possible association between infections of humans with intestinal parasitic helminths and allergies.
van den Biggelaar, A. H. et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 356, 1723–1727 (2000).
Araujo, M. I. et al. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int. Arch. Allergy Immunol. 123, 145–148 (2000). References 37 and 38 are the first formal demonstrations of associations between infections of humans with schistosomes and lowered allergic responses.
Medeiros, M. Jr. et al. Schistosoma mansoni infection is associated with a reduced course of asthma. J. Allergy Clin. Immunol. 111, 947–951 (2003).
Loke, P. et al. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 3, 7 (2002).
Voehringer, D., Shinkai, K. & Locksley, R. M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20, 267–277 (2004).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Owyang, A. M. et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849 (2006).
Pesce, J. et al. The IL-21 receptor augments TH2 effector function and alternative macrophage activation. J. Clin. Invest. 116, 2044–2055 (2006).
Dong, C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nature Rev. Immunol. 6, 329–333 (2006).
Hunter, C. A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nature Rev. Immunol. 5, 521–531 (2005).
Vella, A. T. & Pearce, E. J. CD4+ TH2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, TH0-like stage. J. Immunol. 148, 2283–2290 (1992).
Fallon, P. G., Emson, C. L., Smith, P. & McKenzie, A. N. IL-13 overexpression predisposes to anaphylaxis following antigen sensitization. J. Immunol. 166, 2712–2716 (2001).
Mangan, N. E. et al. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 173, 6346–6356 (2004).
Bashir, M. E., Andersen, P., Fuss, I. J., Shi, H. N. & Nagler-Anderson, C. An enteric helminth infection protects against an allergic response to dietary antigen. J. Immunol. 169, 3284–3292 (2002). References 49 and 50 describe a novel IL-10-mediated and B-cell-mediated mechanism, whereby schistosomes render mice refractory to experimental anaphylaxis.
Strait, R. T., Morris, S. C., Smiley, K., Urban, J. F. Jr. & Finkelman, F. D. IL-4 exacerbates anaphylaxis. J. Immunol. 170, 3835–3842 (2003).
Richards, I. M. et al. Ascaris-induced bronchoconstriction in primates experimentally infected with Ascaris suum ova. Clin. Exp. Immunol. 54, 461–468 (1983).
Kamradt, T., Goggel, R. & Erb, K. J. Induction, exacerbation and inhibition of allergic and autoimmune diseases by infection. Trends Immunol. 26, 260–267 (2005).
Stock, P. et al. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nature Immunol. 5, 1149–1156 (2004).
Stock, P., DeKruyff, R. H. & Umetsu, D. T. Inhibition of the allergic response by regulatory T cells. Curr. Opin. Allergy Clin. Immunol. 6, 12–16 (2006).
Hawrylowicz, C. M. & O'Garra, A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nature Rev. Immunol. 5, 271–283 (2005).
Akdis, M. et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199, 1567–1575 (2004). This paper shows evidence in humans of reciprocal roles for T H 2 versus regulatory T cells in allergic versus healthy individuals.
Francis, J. N., Till, S. J. & Durham, S. R. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J. Allergy Clin. Immunol. 111, 1255–1261 (2003).
Jutel, M. et al. IL-10 and TGF-β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur. J. Immunol. 33, 1205–1214 (2003).
Xystrakis, E. et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Invest. 116, 146–155 (2006).
Doetze, A. et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by TH3/Tr1-type cytokines IL-10 and transforming growth factor-β but not by a TH1 to TH2 shift. Int. Immunol. 12, 623–630 (2000).
Babu, S., Blauvelt, C. P., Kumaraswami, V. & Nutman, T. B. Regulatory networks induced by live parasites impair both TH1 and TH2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 176, 3248–3256 (2006).
van der Kleij, D. et al. A novel host–parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277, 48122–48129 (2002). In this paper a comprehensive biochemical and immunological analysis was carried out to determine the immunomodulatory activity of a newly identified lipid from schistosomes.
Wang, C. C., Nolan, T. J., Schad, G. A. & Abraham, D. Infection of mice with the helminth Strongyloides stercoralis suppresses pulmonary allergic responses to ovalbumin. Clin. Exp. Allergy 31, 495–503 (2001).
Wohlleben, G. et al. Helminth infection modulates the development of allergen-induced airway inflammation. Int. Immunol. 16, 585–596 (2004). This study is the first to demonstrate of a role for IL-10 in suppression of experimental allergic lung inflammation by a parasitic nematode. The mechanism was confirmed to involve CD4+ T cells cells in references 66 and 67.
Wilson, M. S. et al. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202, 1199–1212 (2005).
Kitagaki, K. et al. Intestinal helminths protect in a murine model of asthma. J. Immunol. 177, 1628–1635 (2006).
Mangan, N. E., van Rooijen, N., McKenzie, A. N. & Fallon, P. G. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J. Immunol. 176, 138–147 (2006). This paper describes the first experimental evidence that schistosome infection induces a modified type 2 pulmonary response, in which infected mice are resistant to allergic lung inflammation.
Mizoguchi, A. & Bhan, A. K. A case for regulatory B cells. J. Immunol. 176, 705–710 (2006).
Lau, S. et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Lancet 356, 1392–1397 (2000).
Platts-Mills, T., Vaughan, J., Squillace, S., Woodfolk, J. & Sporik, R. Sensitisation, asthma, and a modified TH2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet 357, 752–756 (2001).
Erwin, E. A. et al. Cat and dust mite sensitivity and tolerance in relation to wheezing among children raised with high exposure to both allergens. J. Allergy Clin. Immunol. 115, 74–79 (2005).
Platts-Mills, T. A., Woodfolk, J. A., Erwin, E. A. & Aalberse, R. Mechanisms of tolerance to inhalant allergens: the relevance of a modified TH2 response to allergens from domestic animals. Springer Semin. Immunopathol. 25, 271–279 (2004). References 72 and 73 review the modified T H 2 response induced by cat allergens.
Lau, S. et al. Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitization, cat-specific IgG and development of asthma in childhood — report of the German Multicentre Allergy Study (MAS 90). Allergy 60, 766–773 (2005).
Reefer, A. J. et al. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified TH2 response to cat allergen. J. Immunol. 172, 2763–2772 (2004).
Carneiro, R. et al. T cell epitope-specific defects in the immune response to cat allergen in patients with atopic dermatitis. J. Invest. Dermatol. 122, 927–936 (2004).
Demeure, C. E. et al. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J. Infect. Dis. 168, 1000–1008 (1993).
Smithers, S. R. & Terry, R. J. Resistance to experimental infection with Schistosoma mansoni in rhesus monkeys induced by the transfer of adult worms. Trans. R. Soc. Trop. Med. Hyg. 61, 517–533 (1967).
Brown, S. P. & Grenfell, B. T. An unlikely partnership: parasites, concomitant immunity and host defence. Proc. Biol. Sci. 268, 2543–2549 (2001).
Wilson, R. A. Leaky livers, portal shunting and immunity to schistosomes. Parasitol. Today 6, 354–358 (1990).
Lambrecht, B. N. & Hammad, H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nature Rev. Immunol. 3, 994–1003 (2003).
Weinstock, J. V., Summers, R. W. & Elliott, D. E. Role of helminths in regulating mucosal inflammation. Springer Semin. Immunopathol. 27, 249–271 (2005). This paper reviews the modulation of inflammatory bowel disease by helminths and outlines potential immunological mechanisms. References 84–86 describe clinical helminth infection of patients with IBD.
Macdonald, T. T. & Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 307, 1920–1925 (2005).
Summers, R. W., Elliott, D. E., Urban, J. F. Jr, Thompson, R. & Weinstock, J. V. Trichuris suis therapy in Crohn's disease. Gut 54, 87–90 (2005).
Summers, R. W., Elliott, D. E., Urban, J. F. Jr, Thompson, R. A. & Weinstock, J. V. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128, 825–832 (2005).
Croese, J. et al. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut 55, 136–137 (2006).
Falcone, F. H. & Pritchard, D. I. Parasite role reversal: worms on trial. Trends Parasitol. 21, 157–160 (2005). This is a review of the therapeutic potential of helminths. Reference 88 provides a broader review of the use of molecules from various pathogens as immunotherapeutics.
Fallon, P. G. & Alcami, A. Pathogen-derived immunomodulatory molecules: future immunotherapeutics? Trends Immunol. 27, 470–476 (2006).
Harnett, W., McInnes, I. B. & Harnett, M. M. ES-62, a filarial nematode-derived immunomodulator with anti-inflammatory potential. Immunol. Lett. 94, 27–33 (2004).
Thomas, P. G. & Harn, D. A. Jr. Immune biasing by helminth glycans. Cell Microbiol. 6, 13–22 (2004).
Smith, P. et al. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J. Exp. Med. 202, 1319–1325 (2005).
Donnelly, S., O'Neill, S. M., Sekiya, M., Mulcahy, G. & Dalton, J. P. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 73, 166–173 (2005).
Maizels, R. M. et al. Helminth parasites — masters of regulation. Immunol. Rev. 201, 89–116 (2004). This is a broad review of the range of regulatory activity of helminths.
Hesse, M. et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 172, 3157–3166 (2004).
McKee, A. S. & Pearce, E. J. CD25+CD4+ cells contribute to TH2 polarization during helminth infection by suppressing TH1 response development. J. Immunol. 173, 1224–1231 (2004).
Taylor, M. D. et al. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 174, 4924–4933 (2005).
Wynn, T. A. et al. IL-10 regulates liver pathology in acute murine Schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J. Immunol. 160, 4473–4480 (1998).
Hoffmann, K. F., Cheever, A. W. & Wynn, T. A. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164, 6406–6416 (2000).
Velupillai, P., Secor, W. E., Horauf, A. M. & Harn, D. A. B-1 cell (CD5+B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J. Immunol. 158, 338–344 (1997).
Jankovic, D. et al. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. J. Exp. Med. 187, 619–629 (1998).
Baumgart, M., Tompkins, F., Leng, J. & Hesse, M. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 176, 5374–5387 (2006).
Taylor, J. J., Mohrs, M. & Pearce, E. J. Regulatory T cell responses develop in parallel to TH responses and control the magnitude and phenotype of the TH effector population. J. Immunol. 176, 5839–5847 (2006).
MacDonald, A. S., Straw, A. D., Bauman, B. & Pearce, E. J. CD8− dendritic cell activation status plays an integral role in influencing TH2 response development. J. Immunol. 167, 1982–1988 (2001).
Thomas, P. G. et al. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J. Immunol. 171, 5837–5841 (2003).
Balic, A., Harcus, Y., Holland, M. J. & Maizels, R. M. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives TH2 immune responses. Eur. J. Immunol. 34, 3047–3059 (2004).
Goodridge, H. S. et al. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology 113, 491–498 (2004).
Jankovic, D., Kullberg, M. C., Caspar, P. & Sher, A. Parasite-induced TH2 polarization is associated with down-regulated dendritic cell responsiveness to TH1 stimuli and a transient delay in T lymphocyte cycling. J. Immunol 173, 2419–2427 (2004).
Mallevaey, T. et al. Activation of invariant NKT cells by the helminth parasite Schistosoma mansoni. J. Immunol. 176, 2476–2485 (2006).
Atochina, O., Daly-Engel, T., Piskorska, D., McGuire, E. & Harn, D. A. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1+ macrophages that suppress naive CD4+ T cell proliferation via an IFN-γ and nitric oxide-dependent mechanism. J. Immunol. 167, 4293–4302 (2001).
Smith, P. et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J. Immunol. 173, 1240–1248 (2004).
Loke, P., MacDonald, A. S., Robb, A., Maizels, R. M. & Allen, J. E. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur. J. Immunol. 30, 2669–2678 (2000).
Taylor, M. D., Harris, A., Nair, M. G., Maizels, R. M. & Allen, J. E. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J. Immunol. 176, 6918–6927 (2006).
Nair, M. G. et al. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect. Immun. 73, 385–394 (2005).
Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 (2001).
Herbert, D. R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004).
Anthony, R. M. et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 12, 955–960 (2006). This study demonstrates the interplay between T H 2 cells and alternatively activated macrophages in parasitic nematode infection.
King, C. H., Dickman, K. & Tisch, D. J. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet 365, 1561–1569 (2005).
Fallon, P. G., Richardson, E. J., Smith, P. & Dunne, D. W. Elevated type 1, diminished type 2 cytokines and impaired antibody response are associated with hepatotoxicity and mortalities during Schistosoma mansoni infection of CD4-depleted mice. Eur. J. Immunol. 30, 470–480 (2000).
Karanja, D. M., Colley, D. G., Nahlen, B. L., Ouma, J. H. & Secor, W. E. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am. J. Trop. Med. Hyg. 56, 515–521 (1997).
Pearce, E. J. & MacDonald, A. S. The immunobiology of schistosomiasis. Nature Rev. Immunol. 2, 499–511 (2002). This is an authorative general review on schistosomiasis, which is also reviewed in references 121 and 122.
Wynn, T. A., Thompson, R. W., Cheever, A. W. & Mentink-Kane, M. M. Immunopathogenesis of schistosomiasis. Immunol. Rev. 201, 156–167 (2004).
Fallon, P. G. Immunopathology of schistosomiasis: a cautionary tale of mice and men. Immunol. Today 21, 29–35 (2000).
Okano, M., Satoskar, A. R., Nishizaki, K. & Harn, D. A. Jr. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing TH2-type response. J. Immunol. 167, 442–450 (2001).
Smith, P. et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. (in the press).
Acknowledgements
The authors are currently supported by the Irish Higher Education Authority Programme for Research in Third Level Institutions, with previous support from SFI and The Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome
FURTHER INFORMATION
Glossary
- Allergen
-
An antigenic substance that can induce an allergic response.
- Anthelminthic
-
A drug that is used to kill parasitic helminths.
- Geohelminth
-
A parasitic helminth in which part of the life cycle involves living in the soil.
- Nematode
-
A phylum of worms with elongated bodies that include free-living species (such as Caenorhabditis elegans) and parasitic species.
- Filarial worm
-
A parasitic nematode, the life cycle of which involves an invertebrate intermediate host and a vertebrate primary host.
- Trematode
-
A class of parasitic flatworms that is often referred to as the flukes, and includes schistosome species in the blood.
- Granuloma
-
A localized cell infiltration surrounding, in this context, a schistosome egg trapped in various tissues. The schistosome egg granuloma comprises a type 2 infiltrate that is rich in TH2 cells, eosinophils and macrophages, which progresses to a fibrotic lesion.
- Cercariae
-
Infective free-swimming larvae of trematodes. Humans are infected when the cercariae penetrate the skin while the individual is in contaminated water.
- Airway hyperresponsiveness
-
A hyperreactivity of the airways, initiated by exposure to a defined stimulus that is usually tolerated by normal individuals, that causes bronchoconstriction and inflammatory-cell infiltration in allergic individuals.
- Airway remodelling
-
Chronic changes in the airway architecture of the lungs of allergic individuals that contribute to airway hyperresponsiveness.
Rights and permissions
About this article
Cite this article
Fallon, P., Mangan, N. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol 7, 220–230 (2007). https://doi.org/10.1038/nri2039
Issue Date:
DOI: https://doi.org/10.1038/nri2039
This article is cited by
-
Antigenic cross-reactivity between Schistosoma mansoni and allergenic invertebrates putatively due to shared glycanic epitopes
Scientific Reports (2020)
-
Immunomodulatory effect of different extracts from Angiostrongylus cantonensis on airway inflammation in an allergic asthma model
Parasitology Research (2020)
-
Absence of Batf3 results in reduced liver pathology in mice infected with Schistosoma japonicum
Parasites & Vectors (2017)
-
Changes in protein expression after treatment with Ancylostoma caninum excretory/secretory products in a mouse model of colitis
Scientific Reports (2017)
-
Effects of poor hygiene on cytokine phenotypes in children in the tropics
World Allergy Organization Journal (2016)