Key Points
-
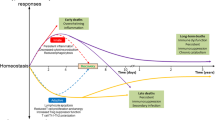
Sepsis is the systemic inflammatory response that occurs following severe infections and is characterized by a range of features, which might include fever, hypotension, altered mental status and shortness of breath.
-
Although the predominant theory has been that the death of patients with sepsis was due to an over-exuberant inflammatory response, it is now apparent that many deaths are due to failure of the host to mount an effective immunological response. As the sepsis progresses, patients develop a state of immunoparalysis, marked by an inability of the host to eradicate the invading pathogen and predisposition to secondary infections.
-
A major cause of the immunoparalysis of sepsis is the loss of key immune effector cells, including dendritic cells and lymphocytes. These cells die owing to sepsis-induced apoptosis.
-
Uptake of apoptotic cells by professional scavenging cells induces a T helper 2 (TH2) phenotype or anergy in these phagocytic cells, thereby contributing to the immune suppression.
-
Blockade of sepsis-induced apoptosis by a number of methods, including overexpression of B-cell lymphoma 2 (BCL-2) or AKT results in improved survival. This finding suggests that apoptosis is an important process in the pathophysiology of the disorder.
-
It is now clear that caspases have other functions in the immune system in addition to their role as cell-death proteases. Caspases might also regulate inflammation, cellular activation and cellular proliferation.
-
Strategies to block sepsis-induced apoptosis might represent a novel therapy of this highly lethal disorder.
Abstract
Although the prevailing concept has been that mortality in sepsis results from an unbridled hyper-inflammatory cytokine-mediated response, the failure of more than 30 clinical trials to treat sepsis by controlling this cytokine response requires a 'rethink' of the molecular mechanism underpinning the development of sepsis. As we discuss here, remarkable new studies indicate that most deaths from sepsis are actually the result of a substantially impaired immune response that is due to extensive death of immune effector cells. Rectification of this apoptotic–inflammatory imbalance using modulators of caspases and other components of the cell-death pathway have shown striking efficacy in stringent animal models of sepsis, indicating an entirely novel path forward for the clinical treatment of human sepsis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bone, R. C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101, 1644–1655 (1992).
Angus, D. C. et al. Epidemiology of severe sepsis in the United States. Crit. Care Med. 29, 1303–1310 (2001).
Murphy, S. L. Deaths: final data for 1998. Natl Vital Stat. Rep. 48, 1–105 (1998).
Deitch, E. A. Animal models of sepsis and shock: a review and lessons learned. Shock 9, 1–11 (1998).
O'Reilly, M., Newcomb, D. E. & Remick, D. Endotoxin, sepsis, and the primrose path. Shock 12, 411–420 (1999).
Fisher, C. J. et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N. Engl. J. Med. 334, 1697–1702 (1996). Shows that blocking TNF using a receptor fusion protein in patients with septic shock did not reduce mortality and at higher doses seems to worsen the outcome.
Zeni, F., Freeman, B. & Natanson, C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit. Care Med. 25, 1095–1100 (1997).
Oberholzer, A., Oberholzer, C. & Moldawer, L. L. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16, 83–96 (2001).
Ertel, W. et al. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 85, 1341–1347 (1995).
Docke, W. D. et al. Monocyte deactivation in septic patients: restoration by IFN-γ treatment. Nature Med. 3, 678–681 (1997). Describes the first successful therapy of a subset of patients with sepsis using a pro-inflammatory cytokine (IFNγ).
Monneret, G. How to identify systemic sepsis-induced immunoparalysis. Adv. Sepsis 4, 42–49 (2005).
Hotchkiss, R. S. & Karl, I. E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 (2003).
Hotchkiss, R. S. et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 27, 1230–1251 (1999). This is the first study to document that apoptosis is an important mechanism of cell death and immune-cell depletion in patients with sepsis.
Hiramatsu, M., Hotchkiss, R. S., Karl, I. E. & Buchman, T. G. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 7, 247–253 (1997).
Chung, C. S., Xu, Y. X., Wang, W., Chaudry, I. H. & Ayala, A. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis? Arch. Surg. 133, 1213–1220 (1998).
Oberholzer, C. et al. Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc. Natl Acad. Sci. USA 98, 11503–11508 (2001).
Hotchkiss, R. S. et al. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit. Care Med. 25, 1298–1307 (1997).
Oberholzer, C., Oberholzer, A., Clare-Salzler, M. & Moldawer, L. L. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 15, 879–892 (2001).
Coopersmith, C. et al. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 287, 1716–1721 (2002).
Kinloch, R. A., Treherne, J. M., Furness, L. M. & Hajimohamadreza, I. The pharmacology of apoptosis. Trends Pharmacol. Sci. 20, 35–42 (1999).
Hotchkiss, R. S. et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166, 6952–6963 (2001).
Hotchkiss, R. S. et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J. Immunol. 168, 2493–2500 (2002).
Felmet, K. A., Hall, M. W., Clark, R. S., Jaffe, R. & Carcillo, J. A. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J. Immunol. 174, 3765–3772 (2005).
Toti, P. et al. Spleen depletion in neonatal sepsis and chorioamnionitis. Am. J. Clin. Pathol. 122, 765–771 (2004).
Le Tulzo, Y. et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 18, 487–494 (2002).
Hotchkiss, R. S. et al. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. 174, 5110–5118 (2005).
Fearon, D. T. & Locksley, R. M. The instructive role of innate immunity in the acquired immune response. Science 272, 50–53 (1996).
Lederer, J. A., Rodrick, M. L. & Mannick, J. A. The effects of injury on the adaptive immune response. Shock 11, 153–159 (1999).
Oberholzer, A., Oberholzer, C. & Moldawer, L. L. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16, 83–96 (2001).
Griffith, T. S., Yu, X., Herndon, J. M., Green, D. R. & Ferguson, T. A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity 5, 7–16 (1996). The first paper to show that apoptotic cells induce an immunological tolerant state in vivo.
Voll, R. E. et al. Immunosuppressive effects of apoptotic cells. Nature 390, 350–351 (1997).
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 (1998).
Green, D. R. & Beere, H. M. Gone but not forgotten. Nature 405, 28–29 (2000).
Albert, M. L. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nature Rev. Immunol. 4, 223–231 (2004).
Gogos, C. A., Drosou, E., Bassaris, H. P. & Skoutelis, A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J. Infect. Dis. 181, 176–180 (2000).
van Dissel, J. T., van Langevelde, P., Westendorp, R. G., Kwappenberg, K. & Frolich, M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351, 950–953 (1998). A large study of patients admitted with fever, which shows that a high ratio of IL-10 to TNF was associated with a fatal outcome. The authors warned against a widespread use of pro-inflammatory cytokine-inhibition in patients with sepsis.
Monneret, G. et al. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol. Lett. 95, 193–198 (2004).
Hotchkiss, R. S. et al. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc. Natl Acad. Sci. USA 100, 6724–6729 (2003).
Roy, S. & Nicholson, D. W. Cross-talk in cell death signalling. J. Expl. Med. 192, F21–F25 (2000).
Marsden, V. S. & Strasser, A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 21, 71–105 (2003).
Strasser, A. The role of BH3-only proteins in the immune system. Nature Rev. Immunol. 5, 189–200 (2005).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Gupta, S. Molecular mechanisms of apoptosis in the cells of the immune system in human aging. Immunol. Rev. 205, 114–129 (2005).
Hotchkiss, R. S. et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162, 4148–4156 (1999). The first study to show that blocking apoptosis improves survival in sepsis.
Yin, X. M. et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 (1999).
Creagh, E. M. & Martin, S. J. Caspases: cellular demolition experts. Biochem. Soc. Trans. 29, 696–702 (2001).
Perfettini, J. L. & Kroemer, G. Caspase activation is not death. Nature Immunol. 4, 308–310 (2003).
Newton, K. & Strasser, A. Caspases signal not only apoptosis but also antigen-induced activation in cells of the immune system. Genes Dev. 17, 819–825 (2003).
Creagh, E. M., Conroy, H. & Martin, S. J. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 193, 10–21 (2003).
Chung, C. S. et al. Inhibition of Fas signaling prevents hepatic injury and improves organ blood flow during sepsis. Surgery 130, 339–345 (2001).
Chung, C. S. et al. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J. Leukoc. Biol. 74, 344–351 (2003).
Wesche-Soldato, D. E. et al. In vivo delivery of caspase 8 or Fas siRNA improves the survival of septic mice. Blood 106, 2295–2301 (2005). This study shows that anti-apoptotic therapy that is based on siRNA improves survival in sepsis.
Chang, K. C. et al. Multiple triggers of cell death in sepsis — death receptor and mitochondrial-mediated apoptosis. FASEB J. (in the press).
Efron, P. A. et al. Increased lymphoid tissue apoptosis in baboons with bacteremic shock. Shock 21, 566–571 (2004).
Nicholson, D. W. et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376, 37–43 (1995). This study identifies the mammalian caspase-3, showed that it was related to IL-1β-converting enzyme inhibitor, and shows that it was essential for mammalian apoptosis. The authors also developed potent peptide aldehyde inhibitors that prevented apoptosis.
Nicholson, D. W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6, 1028–1042 (1999).
Martinon, F. & Tschopp, J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117, 561–574 (2004).
Martin, S. J. & Green, D. R. Protease activation during apoptosis: death by a thousand cuts? Cell 82, 349–352 (1995).
Lancel, S. et al. Ventricular myocyte caspases are directly responsible for endotoxin-induced cardiac dysfunction. Circulation 111, 2596–2604 (2005).
Carlson, D. L., Willis, M. S., White, D. J., Horton, J. W. & Giroir, B. P. Tumor necrosis factor-α-induced caspase activation mediates endotoxin-related cardiac dysfunction. Crit. Care Med. 33, 1021–1028 (2005).
Parrillo, J. E. Pathogenetic mechanisms of septic shock. N. Engl. J. Med. 328, 1471–1477 (1993).
Neviere, R., Fauvel, H., Chopin, C., Formstecher, P. & Marchetti, P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am. J. Respir. Crit. Care Med. 163, 218–225 (2001).
Kawasaki, M. et al. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am. J. Pathol. 157, 597–603 (2000).
Guo, R., Wang, Y., Minto, A. W., Quigg, R. J. & Cunningham, P. N. Acute renal failure in endotoxemia is dependent on caspase activation. J. Am. Soc. Nephrol. 15, 3093–3102 (2004).
Morishima, N., Nakanishi, K., Takenouchi, H., Shibata, T. & Yasuhiko, Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277, 34287–34294 (2002).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Saleh, M. et al. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429, 75–79 (2004). Shows that polymorphisms in human caspase-12 regulate the intensity of the inflammatory response and might explain differences in the morbidity and mortality in selected populations with sepsis.
Saleh, M. et al. Enhanced bacterial clearance and sepsis resistance in caspase-12 deficient mice. Nature 440, 1064–1068. (2006).
Iwata, A. et al. Over-expression of Bcl-2 provides protection in septic mice by a trans effect. J. Immunol. 171, 3136–3141 (2003).
Haendeler, J., Messmer, U. K., Brune, B., Neugebauer, E. & Dimmeler, S. Endotoxic shock leads to apoptosis in vivo and reduces Bcl-2. Shock 6, 405–409 (1996).
Bilbault, P. et al. Transient Bcl-2 gene down-expression in circulating mononuclear cells of severe sepsis patients who died despite appropriate intensive care. Intensive Care Med. 30, 408–415 (2004).
Bommhardt, U. et al. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J. Immunol. 172, 7583–7591 (2004).
Wesche, D. E., Lomas-Neira, J. L., Perl, M., Chung, C. S. & Ayala, A. Leukocyte apoptosis and its significance in sepsis and shock. J. Leukoc. Biol. 78, 325–337 (2005).
Braun, J. S. et al. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nature Med. 5, 298–302 (1999).
Hotchkiss, R. S. et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl Acad. Sci. USA 96, 14541–14546 (1999).
Hotchkiss, R. S. et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nature Immunol. 1, 496–501 (2000).
Methot, N. et al. Differential efficacy of caspase inhibitors on apoptosis markers during sepsis in rats and implication for fractional inhibition requirements for therapeutics. J. Exp. Med. 199, 199–207 (2004).
Chun, H. J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002).
Kang, T. B. et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J. Immunol. 173, 2976–2984 (2004).
Sordet, O. et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood 100, 4446–4453 (2002).
Olson, N. E., Graves, J. D., Shu, G. L., Ryan, E. J. & Clark, E. A. Caspase activity is required for stimulated B lymphocytes to enter the cell cycle. J. Immunol. 170, 6065–6072 (2003).
Opferman, J. T. & Korsmeyer, S. J. Apoptosis in the development and maintenance of the immune system. Nature Immunol. 4, 410–415 (2003).
Su, H. et al. Requirement for caspase-8 in NF-κB activation by antigen receptor. Science 307, 1465–1468 (2005).
Wong, S. H., Santambrogio, L. & Strominger, J. L. Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins. Proc. Natl Acad. Sci. USA 101, 17783–17788 (2004).
Cauwels, A., Janssen, B., Waeytens, A., Cuvelier, C. & Brouckaert, P. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nature Immunol. 4, 387–393 (2003).
Kroemer, G. & Martin, S. J. Caspase-independent cell death. Nature Med. 11, 725–730 (2005).
Jaattela, M. & Tschopp, J. Caspase-independent cell death in T lymphocytes. Nature Immunol. 4, 416–423 (2003).
Chipuk, J. E. & Green, D. R. Do inducers of apoptosis trigger caspase-independent cell death? Nature Rev. Mol. Cell Biol. 6, 268–275 (2005).
Green, D. R. & Kroemer, G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004).
Cregan, S. P. et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J. Cell Biol. 158, 507–517 (2002).
Leist, M. & Jaattela, M. Four deaths and a funeral: from caspases to alternative mechanisms. Nature Rev. Mol. Cell Biol. 2, 589–598 (2001).
Phenix, B. N., Cooper, C., Owen, C. & Badley, A. D. Modulation of apoptosis by HIV protease inhibitors. Apoptosis 7, 295–312 (2002).
Estaquier, J. et al. Effects of antiretroviral drugs on human immunodeficiency virus type 1-induced CD4+ T-cell death. J. Virol. 76, 5966–5973 (2002).
Phenix, B. N., Lum, J. J., Nie, Z., Sanchez-Dardon, J. & Badley, A. D. Antiapoptotic mechanism of HIV protease inhibitors: preventing mitochondrial transmembrane potential loss. Blood 98, 1078–1085 (2001).
Weaver, J. G., Rouse, M. S., Steckelberg, J. M. & Badley, A. D. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 18, 1185–1191 (2004).
Weaver, J. G. et al. Inhibition of adenine nucleotide translocator pore function and protection against apoptosis in vivo by an HIV protease inhibitor. J. Clin. Invest. 115, 1828–1838 (2005).
Schwab, B. L. et al. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ. 9, 818–831 (2002).
Kim, R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer 103, 1551–1560 (2005).
Jaattela, M. Programmed cell death: many ways for cells to die decently. Ann. Med. 34, 480–488 (2002).
Cheng, T. et al. Activated protein C blocks p53-mediated apoptosis in ischemic brain endothelium and is neuroprotective. Nature Med. 9, 258–260 (2003).
Nicholson, D. W. From bench to clinic with apoptosis-based therapeutic agents. Nature 407, 810–816 (2000).
Akira, A, Uematsu, S. & Takeuchi O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Conte, D. et al. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol. Cell. Biol. 26, 699–708 (2006).
Schwarze, S. R., Ho, A., Vocero-Akbani, A. & Dowdy, S. F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999). The first study to show that large macromolecular cargoes could be delivered intracellularly if conjugated to permeation peptides that are derived from the Tat-basic domain.
Kilic, E., Dietz, G. P., Hermann, D. M. & Bahr, M. Intravenous TAT–Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann. Neurol. 52, 617–622 (2002).
Cao, G. et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J. Neurosci. 22, 5423–5431 (2002).
Sugioka, R. et al. BH4-domain peptide from Bcl-xL exerts anti-apoptotic activity in vivo. Oncogene 22, 8432–8440 (2003).
Denicourt, C. & Dowdy, S. F. Protein transduction technology offers novel therapeutic approach for brain ischemia. Trends Pharmacol. Sci. 24, 216–218 (2003).
Hotchkiss, R. S., et al. TAT–BH4 and TAT–Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J. Immunol. 176, 5471–5477 (2006).
Acknowledgements
Work from the Hotchkiss laboratory was supported by grants from the US National Institutes of Health, and the Alan A. and Edith L. Wolff Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Endotoxin
-
Endotoxin is the lipopolysaccharide component that constitutes the cell wall of Gram-negative bacteria. It is a potent activator of many cells including monocytes, macrophages and B cells. During sepsis induced by Gram-negative bacteria, endotoxin is released into the bloodstream thereby resulting in many of the clinical signs and symptoms of sepsis.
- Apoptosis
-
Apoptosis involves cell shrinkage, chromatin condensation in the periphery of the nucleus, cell-membrane blebbing and DNA fragmentation into multiples of ∼180 base pairs. Eventually, the cell breaks up into many membrane-bound apoptotic bodies, which are phagocytosed by neighbouring cells.
- Caspases
-
A family of cysteine proteinases that are involved in the initiation and effector stages of apoptosis.
- Follicular dendritic cells
-
(FDCs). Cells with a dendritic morphology that are present in the B-cell areas of spleen and lymph nodes. FDCs have intact antigens on their surface that are held in immune complexes, and B cells present in the lymph node can interact with these antigen-presenting cells. FDCs are of non-haematopoietic origin and are not related to other types of dendritic cell.
- Interdigitating dendritic cells
-
A potent antigen-presenting cell that is rich in MHC class II molecules. Interdigitating dendritic cells take up antigen in the periphery and migrate to the paracortical region of lymph nodes and spleen where they interact with T cells.
- Septic shock
-
Sepsis is the host response that occurs owing to the presence of bacteria and/or their products within the bloodstream. Patients with a severe life-threatening form of sepsis in which there is evidence of inadequate organ perfusion (for example, shock, decreased renal function and depressed mental state) are stated to be in septic shock.
- Lymphopaenia
-
A deficiency of lymphocytes in the blood circulation.
- T helper 2 (TH2)-cell
-
The definition of a CD4+ T cell that has differentiated into a cell that produces the cytokines interleukin-4 (IL-4), IL-5 and IL-13.
- Necrotic cells
-
Cells that are exposed to the high concentrations of purified perforin that are typically delivered by cytolytic cells, such as natural killer cells and cytotoxic T lymphocytes, and usually die by osmotic lysis, a form of necrotic death.
- T helper 1 (TH1)-cell
-
The definition of a CD4+ T cell that has differentiated into a cell that produces the cytokines interferon-γ and tumour-necrosis factor.
- Annexin-V
-
Binds to phosphatidyl serine, which is normally located on the inner leaflet of the plasma membrane, but which flips to the outer layer during apoptosis. Annexin-V staining is often used as an indicator of apoptosis.
- TUNEL
-
An in situ method for detecting the 3′-OH ends of DNA that are exposed during the internucleosomal cleavage that occurs during apoptosis.
- Small interfering RNAs
-
Synthetic double-stranded RNA molecules of 19–23 nucleotides, which are used to 'knock down' (silence the expression of) a specific gene. This is known as RNA interference (RNAi) and is mediated by the sequence-specific degradation of mRNA.
- Myocardial depression
-
Patients with sepsis often have significantly decreased heart contractility that might be manifested by decreased blood pressure or shock. This decrease in heart function during sepsis is termed myocardial depression and is thought to be due, in part, to high circulating concentrations of pro-inflammatory cytokines, including tumour-necrosis factor.
- AKT
-
(Also known as protein kinase B). A key component of the phosphatidylinositol 3-kinase signalling pathway. AKT has potent anti-apoptotic activity, and T cells that overexpress AKT are resistant to sepsis-induced apoptosis.
- Cecal ligation and puncture
-
(CLP). CLP is a standard animal model used to induce sepsis. In the CLP model, the cecum, a large sac-like structure that is located at the junction of the small and large intestine, is occluded by suture ligation and punctured with a needle. During the subsequent time period, the animal develops intra-abdominal polymicrobial infection. The CLP model reproduces many of the signs and symptoms of sepsis that occur in patients.
Rights and permissions
About this article
Cite this article
Hotchkiss, R., Nicholson, D. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6, 813–822 (2006). https://doi.org/10.1038/nri1943
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1943
This article is cited by
-
Risk of malignant disease in 1-year sepsis survivors, a registry-based nationwide follow-up study
Critical Care (2023)
-
Genome-wide identification and functional analysis of dysregulated alternative splicing profiles in sepsis
Journal of Inflammation (2023)
-
Role of dexmedetomidine in modifying immune paralysis in patients with septic shock: randomized controlled trial
Intensive Care Medicine Experimental (2023)
-
Cell-free DNA as diagnostic and prognostic biomarkers for adult sepsis: a systematic review and meta-analysis
Scientific Reports (2023)
-
Risk factors for progression of Urolith Associated with Obstructive Urosepsis to severe sepsis or septic shock
BMC Urology (2022)