Key Points
-
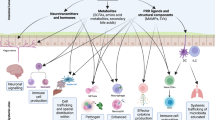
Pathogens have to overcome colonization resistance by the microbiota to colonize the gut and to cause disease.
-
The microbiota modulates the immune system to limit pathogen colonization but also inadvertently helps certain pathogens to colonize, for example, by making electron acceptors and carbon sources available.
-
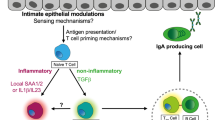
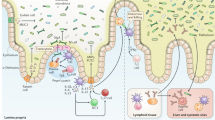
Pathogens are quickly sensed by innate pattern-recognition receptors (for example, Toll-like receptors (TLRs) and NOD-like receptors (NLRs)) on various cell types, which results in a pro-inflammatory response, for example, activation of the inflammasome.
-
Activation of innate receptors triggers an inflammatory response (for example, the interleukin-23–T helper 17 cell axis) that for some pathogens initially promotes colonization but ultimately results in clearance of pathogens.
-
Recruitment of high numbers of neutrophils to the site of infection is a hallmark of inflammatory diarrhoea and is generally beneficial to the host as it controls pathogens.
-
Secretory IgA antibodies are important to maintain the mucosal barrier and to protect against pathogens, for example, Vibrio cholerae or Salmonella spp.
Abstract
The intestinal mucosa is a particularly dynamic environment in which the host constantly interacts with trillions of commensal microorganisms, known as the microbiota, and periodically interacts with pathogens of diverse nature. In this Review, we discuss how mucosal immunity is controlled in response to enteric bacterial pathogens, with a focus on the species that cause morbidity and mortality in humans. We explain how the microbiota can shape the immune response to pathogenic bacteria, and we detail innate and adaptive immune mechanisms that drive protective immunity against these pathogens. The vast diversity of the microbiota, pathogens and immune responses encountered in the intestines precludes discussion of all of the relevant players in this Review. Instead, we aim to provide a representative overview of how the intestinal immune system responds to pathogenic bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Helander, H. F. & Fandriks, L. Surface area of the digestive tract - revisited. Scand. J. Gastroenterol. 49, 681–689 (2014).
Hasleton, P. S. The internal surface area of the adult human lung. J. Anat. 112, 391–400 (1972).
Donnenberg, M. S. & Narayanan, S. How to diagnose a foodborne illness. Infect. Dis. Clin. North Am. 27, 535–554 (2013).
Navaneethan, U. & Giannella, R. A. Mechanisms of infectious diarrhea. Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 637–647 (2008).
Maki, D. G. & Agger, W. A. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Med. (Baltimore) 67, 248–269 (1988).
Kelly, C. P., Pothoulakis, C. & LaMont, J. T. Clostridium difficile colitis. N. Engl. J. Med. 330, 257–262 (1994).
Sassone-Corsi, M. & Raffatellu, M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 194, 4081–4087 (2015).
Buffie, C. G. & Pamer, E. G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 13, 790–801 (2013).
Bohnhoff, M., Drake, B. L. & Miller, C. P. The effect of an antibiotic on the susceptibility of the mouse's intestinal tract to Salmonella infection. Antibiot. Annu. 3, 453–455 (1955).
Kamada, N., Chen, G. Y., Inohara, N. & Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 14, 685–690 (2013).
Winter, S. E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010). This study is the first report of the immune response and the microbiota enhancing the growth of a pathogen; namely, by providing Salmonella spp. with a novel electron acceptor in the inflamed gut.
Lamichhane-Khadka, R., Benoit, S. L., Maier, S. E. & Maier, R. J. A link between gut community metabolism and pathogenesis: molecular hydrogen-stimulated glucarate catabolism aids Salmonella virulence. Open Biol. 3, 130146 (2013).
Maier, R. J., Olczak, A., Maier, S., Soni, S. & Gunn, J. Respiratory hydrogen use by Salmonella enterica serovar Typhimurium is essential for virulence. Infect. Immun. 72, 6294–6299 (2004).
Maier, L. et al. Microbiota-derived hydrogen fuels Salmonella Typhimurium invasion of the gut ecosystem. Cell Host Microbe 14, 641–651 (2013).
Marcobal, A. et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe 10, 507–514 (2011).
Ng, K. M. et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99 (2013). This study shows that disruption of the resident microbiota can alter carbohydrate availability and favour pathogen growth.
Pacheco, A. R. et al. Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117 (2012).
Curtis, M. M. et al. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 16, 759–769 (2014).
Pickard, J. M. et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 514, 638–641 (2014).
Round, J. L. & Mazmanian, S. K. Inducible FOXP3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Kelly, D. et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPARγ and RELA. Nature Immunol. 5, 104–112 (2004).
Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L. & Hooper, L. V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl Acad. Sci. USA 105, 20858–20863 (2008).
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005). The authors report a myeloid-derived mucosal dendritic cell present in the lamina propria that directly samples luminal antigens.
Macpherson, A. J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004). This report shows that dendritic cells carry small numbers of live commensal bacteria, allowing for the selective induction of IgA that protects against commensal microorganism penetration of the mucosal barrier.
Peterson, D. A., McNulty, N. P., Guruge, J. L. & Gordon, J. I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2, 328–339 (2007).
Fagarasan, S. et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 298, 1424–1427 (2002).
Endt, K. et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 6, e1001097 (2010).
Farache, J. et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38, 581–595 (2010).
Ivanov, I. I. et al. Induction of intestinal TH17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009). This study shows that a specific commensal organism, segmented filamentous bacteria (SFB), is sufficient to induce the development of T H 17 cells in the lamina propria.
Goto, Y. et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal TH17 cell differentiation. Immunity 40, 594–607 (2010).
Lecuyer, E. et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 40, 608–620 (2014).
Schnupf, P. et al. Growth and host interaction of mouse segmented filamentous bacteria in vitro. Nature 520, 99–103 (2015).
Ostaff, M. J., Stange, E. F. & Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 5, 1465–1483 (2013).
Salzman, N. H. et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 11, 76–83 (2010). The authors show that the expression of human HD5 in mice modulates the composition of the microbiota, thus playing an important part in regulating commensal microorganism diversity.
Salzman, N. H., Ghosh, D., Huttner, K. M., Paterson, Y. & Bevins, C. L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422, 522–526 (2003).
Chu, H. et al. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science 337, 477–481 (2012).
Wilson, C. L. et al. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286, 113–117 (1999).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Gay, N. J., Symmons, M. F., Gangloff, M. & Bryant, C. E. Assembly and localization of Toll-like receptor signalling complexes. Nat. Rev. Immunol. 14, 546–558 (2014).
O'Neill, L. A., Golenbock, D. & Bowie, A. G. The history of Toll-like receptors — redefining innate immunity. Nat. Rev. Immunol. 13, 453–460 (2013).
Sivick, K. E. et al. Toll-like receptor-deficient mice reveal how innate immune signaling influences Salmonella virulence strategies. Cell Host Microbe 15, 203–213 (2014).
Weiss, D. S., Raupach, B., Takeda, K., Akira, S. & Zychlinsky, A. Toll-like receptors are temporally involved in host defense. J. Immunol. 172, 4463–4469 (2004).
Khan, M. A. et al. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect. Immun. 74, 2522–2536 (2006).
Lebeis, S. L., Bommarius, B., Parkos, C. A., Sherman, M. A. & Kalman, D. TLR signaling mediated by MYD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179, 566–577 (2007).
Gibson, D. L. et al. MYD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10, 618–631 (2008).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Uematsu, S. et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 7, 868–874 (2006).
Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J. & Madara, J. L. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 (2001).
Uematsu, S. & Akira, S. Immune responses of TLR5+ lamina propria dendritic cells in enterobacterial infection. J. Gastroenterol. 44, 803–811 (2009).
Murthy, K. G., Deb, A., Goonesekera, S., Szabo, C. & Salzman, A. L. Identification of conserved domains in Salmonella muenchen flagellin that are essential for its ability to activate TLR5 and to induce an inflammatory response in vitro. J. Biol. Chem. 279, 5667–5675 (2004).
Smith, K. D. et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4, 1247–1253 (2003).
Andersen-Nissen, E. et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl Acad. Sci. USA 102, 9247–9252 (2005).
Gibson, D. L. et al. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10, 388–403 (2008).
Stahl, M. et al. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. PLoS Pathog. 10, e1004264 (2014).
Nishimori, J. H. et al. Microbial amyloids induce interleukin 17A (IL-17A) and IL-22 responses via Toll-like receptor 2 activation in the intestinal mucosa. Infect. Immun. 80, 4398–4408 (2012).
Kanneganti, T. D., Lamkanfi, M. & Nunez, G. Intracellular NOD-like receptors in host defense and disease. Immunity 27, 549–559 (2007).
Fink, S. L. & Cookson, B. T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825 (2006).
Lala, S. et al. Crohn's disease and the NOD2 gene: a role for Paneth cells. Gastroenterology 125, 47–57 (2003).
Eckmann, L. Innate immunity and mucosal bacterial interactions in the intestine. Curr. Opin. Gastroenterol. 20, 82–88 (2004).
Chamaillard, M. et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4, 702–707 (2003).
Girardin, S. E. et al. NOD1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300, 1584–1587 (2003). References 60 and 61 report the recognition of peptidoglycan by NOD1.
Girardin, S. E. et al. NOD2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003).
Inohara, N. et al. NOD1, an APAF1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274, 14560–14567 (1999).
Ogura, Y. et al. NOD2, a NOD1/APAF1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276, 4812–4818 (2001).
LeBlanc, P. M. et al. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe 3, 146–157 (2008).
Kim, Y. G. et al. The NOD2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34, 769–780 (2011).
Geddes, K. et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 17, 837–844 (2011). This study reports the existence of innate T H 17 cells and how their induction is dependent on NOD1 and NOD2 signalling.
Kasper, C. A. et al. Cell-cell propagation of NF-κB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity 33, 804–816 (2010).
Geddes, K. et al. NOD1 and NOD2 regulation of inflammation in the Salmonella colitis model. Infect. Immun. 78, 5107–5115 (2010).
Keestra, A. M. et al. A Salmonella virulence factor activates the NOD1/NOD2 signaling pathway. MBio. 2 (2011).
Keestra, A. M. et al. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature 496, 233–237 (2013).
Srikanth, C. V. et al. Salmonella pathogenesis and processing of secreted effectors by caspase-3. Science 330, 390–393 (2010).
Lee, C. A. et al. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl Acad. Sci. USA 97, 12283–12288 (2000).
Anand, P. K., Malireddi, R. K. & Kanneganti, T. D. Role of the NLRP3 inflammasome in microbial infection. Front. Microbiol. 2, 12 (2011).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via IPAF. Nat. Immunol. 7, 569–575 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006). References 75 and 76 report the discovery of flagellin as the ligand for NLRC4.
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Franchi, L. et al. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat. Immunol. 13, 449–456 (2012).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Liu, Z. et al. Role of inflammasomes in host defense against Citrobacter rodentium infection. J. Biol. Chem. 287, 16955–16964 (2012).
Lebeis, S. L., Powell, K. R., Merlin, D., Sherman, M. A. & Kalman, D. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 77, 604–614 (2009).
Knodler, L. A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Sellin, M. E. et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe 16, 237–248 (2014). References 82 and 83 report that activation of the inflammasome in epithelial cells has a crucial role in antimicrobial defence at the intestinal mucosal surface.
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014). The authors show that the NLRP6 inflammasome is an important regulator of mucin granule exocytosis by goblet cells. Moreover, it links inflammasome signalling to autophagy and highlights the role of goblet cells in host–microbe mutualism.
Godinez, I., Keestra, A. M., Spees, A. & Baumler, A. J. The IL-23 axis in Salmonella gastroenteritis. Cell. Microbiol. 13, 1639–1647 (2011).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Edwards, L. A. et al. Delineation of the innate and adaptive T cell immune outcome in the human host in response to Campylobacter jejuni infection. PLoS ONE 5, e15398 (2010).
Awasthi, A. et al. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 182, 5904–5908 (2009).
Zhou, L. et al. IL-6 programs TH17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and TH17 Cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Kinnebrew, M. A. et al. Interleukin 23 production by intestinal CD103+CD11b+ dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287 (2012).
Raffatellu, M. et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14, 421–428 (2008). In this study, the authors show that SIV infection induces the depletion of T H 17 cells in the ileal mucosa of rhesus macaques, thereby eroding the mucosal barrier to Salmonella spp. dissemination.
Ishigame, H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009).
Malik, A., Sharma, D., St Charles, J., Dybas, L. A. & Mansfield, L. S. Contrasting immune responses mediate Campylobacter jejuni-induced colitis and autoimmunity. Mucosal Immunol. 7, 802–817 (2014).
Kuchta, A. et al. Vibrio cholerae O1 infection induces proinflammatory CD4+ T-cell responses in blood and intestinal mucosa of infected humans. Clin. Vaccine Immunol. 18, 1371–1377 (2011).
Blaschitz, C. & Raffatellu, M. TH17 cytokines and the gut mucosal barrier. J. Clin. Immunol. 30, 196–203 (2010).
Chen, K. et al. TH17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35, 997–1009 (2011).
Monin, L. et al. Immune requirements for protective TH17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 8, 1099–1109 (2015).
Sellge, G. et al. TH17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J. Immunol. 184, 2076–2085 (2010).
Becattini, S. et al. T cell immunity. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science 347, 400–406 (2015). In this paper, the authors find that human antigen-specific memory T cells have different frequencies but comparable diversity, showing that there is a degree of functional heterogeneity in the T cell response.
Rutz, S., Eidenschenk, C. & Ouyang, W. IL-22, not simply a TH17 cytokine. Immunol. Rev. 252, 116–132 (2013).
Behnsen, J. et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 40, 262–273 (2014). In this paper, the authors report a unique role for IL-22 during infection: inducing the expression of antimicrobial proteins that suppress the intestinal microbiota and that favour the growth of a pathogen.
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006). This study identifies the existance of REG3γ, a secreted C-type lectin with antimicrobial activity.
Brandl, K. et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455, 804–807 (2008). In this paper, the authors show that antibiotic treatment of mice downregulates intestinal expression of REG3γ, resulting in decreased killing of vancomycin-resistant Enterococcus spp.
Vaishnava, S. et al. The antibacterial lectin REGIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255–258 (2011).
Fischbach, M. A. et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl Acad. Sci. USA 103, 16502–16507 (2006).
Raffatellu, M. et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5, 476–486 (2009).
Corbin, B. D. et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319, 962–965 (2008). This paper shows that the antimicrobial protein calprotectin functions by sequestering zinc and manganese.
Liu, J. Z. et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11, 227–239 (2012).
Ahlfors, H. et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J. Immunol. 193, 4602–4613 (2014).
Pham, T. A. et al. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe 16, 504–516 (2014).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Mantovani, A., Cassatella, M. A., Costantini, C. & Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Spees, A. M. et al. Neutrophils are a source of γ interferon during acute Salmonella enterica serovar Typhimurium colitis. Infect. Immun. 82, 1692–1697 (2014).
Cua, D. J. & Tato, C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 (2010).
Zindl, C. L. et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl Acad. Sci. USA 110, 12768–12773 (2013).
Taylor, P. R. et al. Activation of neutrophils by autocrine IL-17A–IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat. Immunol. 15, 143–151 (2014).
Sturge, C. R. et al. TLR-independent neutrophil-derived IFNγ is important for host resistance to intracellular pathogens. Proc. Natl Acad. Sci. USA 110, 10711–10716 (2013).
Rydstrom, A. & Wick, M. J. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J. Immunol. 178, 5789–5801 (2007).
Valdez, Y. et al. NRAMP1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell. Microbiol. 11, 351–362 (2009).
Zhang, S. et al. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhea. Infect. Immun. 71, 1–12 (2003).
Noriega, L. M., Van der Auwera, P., Daneau, D., Meunier, F. & Aoun, M. Salmonella infections in a cancer center. Support. Care Cancer 2, 116–122 (1994).
Tumbarello, M., Tacconelli, E., Caponera, S., Cauda, R. & Ortona, L. The impact of bacteraemia on HIV infection. Nine years experience in a large Italian university hospital. J. Infect. 31, 123–131 (1995).
Conlan, J. W. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella Typhimurium. Infect. Immun. 64, 1043–1047 (1996).
Loetscher, Y. et al. Salmonella transiently reside in luminal neutrophils in the inflamed gut. PLoS ONE 7, e34812 (2012).
Perdomo, J. J., Gounon, P. & Sansonetti, P. J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J. Clin. Invest. 93, 633–643 (1994).
Perdomo, O. J. et al. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J. Exp. Med. 180, 1307–1319 (1994).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Zhang, Z., Jin, L., Champion, G., Seydel, K. B. & Stanley, S. L. Jr. Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect. Immun. 69, 3240–3247 (2001).
Spehlmann, M. E. et al. CXCR2-dependent mucosal neutrophil influx protects against colitis-associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J. Immunol. 183, 3332–3343 (2009).
Cerutti, A. & Rescigno, M. The biology of intestinal immunoglobulin A responses. Immunity 28, 740–750 (2008).
Brandtzaeg, P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25, 5467–5484 (2007).
Macpherson, A. J., McCoy, K. D., Johansen, F. E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Bhuiyan, T. R. et al. Cholera caused by Vibrio cholerae O1 induces T-cell responses in the circulation. Infect. Immun. 77, 1888–1893 (2009).
Harris, A. M. et al. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77, 3850–3856 (2009).
Wijburg, O. L. et al. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 203, 21–26 (2006).
Cunningham, A. F. et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 178, 6200–6207 (2007).
MacLennan, C. A. et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 118, 1553–1562 (2008).
Lee, S. J. et al. Identification of a common immune signature in murine and human systemic Salmonellosis. Proc. Natl Acad. Sci. USA 109, 4998–5003 (2012).
Fritz, J. H. et al. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature 481, 199–203 (2011). In this paper, the authors show that mouse IgA+ plasma cells produce inflammatory mediators including TNF and iNOS, revealing that plasma cells adapt to maintain homeostasis in the gut.
Maaser, C. et al. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 72, 3315–3324 (2004).
Kantele, A. et al. Differences in immune responses induced by oral and rectal immunizations with Salmonella Typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun. 66, 5630–5635 (1998).
Qadri, F. et al. Enteric infections in an endemic area induce a circulating antibody-secreting cell response with homing potentials to both mucosal and systemic tissues. J. Infect. Dis. 177, 1594–1599 (1998).
Nachamkin, I. & Yang, X. H. Local immune responses to the Campylobacter flagellin in acute Campylobacter gastrointestinal infection. J. Clin. Microbiol. 30, 509–511 (1992).
McArthur, M. A. et al. Activation of Salmonella Typhi-specific regulatory T cells in typhoid disease in a wild-type S. Typhi challenge model. PLoS Pathog. 11, e1004914 (2015).
Thiagarajah, J. R., Donowitz, M. & Verkman, A. S. Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 12, 446–457 (2015).
Gawenis, L. R. et al. cAMP inhibition of murine intestinal Na/H exchange requires CFTR-mediated cell shrinkage of villus epithelium. Gastroenterology 125, 1148–1163 (2003).
Lin, R. et al. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterology 140, 560–571 (2011).
Walker, N. M. et al. Role of down-regulated in adenoma anion exchanger in HCO3- secretion across murine duodenum. Gastroenterology 136, 893–901 (2009).
Seidler, U. E. Gastrointestinal HCO3- transport and epithelial protection in the gut: new techniques, transport pathways and regulatory pathways. Curr. Opin. Pharmacol. 13, 900–908 (2013).
Mueckler, M. Facilitative glucose transporters. Eur. J. Biochem. 219, 713–725 (1994).
Chen, M. et al. Loss of PDZ-adaptor protein NHERF2 affects membrane localization and cGMP- and [Ca2+]- but not cAMP-dependent regulation of Na+/H+ exchanger 3 in murine intestine. J. Physiol. 588, 5049–5063 (2010).
Yun, C. H. et al. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc. Natl Acad. Sci. USA 94, 3010–3015 (1997).
Field, M., Fromm, D., al-Awqati, Q. & Greenough, W. B. 3rd. Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J. Clin. Invest. 51, 796–804 (1972).
Rao, M. C., Guandalini, S., Smith, P. L. & Field, M. Mode of action of heat-stable Escherichia coli enterotoxin. Tissue and subcellular specificities and role of cyclic GMP. Biochim. Biophys. Acta 632, 35–46 (1980).
Madara, J. L. et al. 5′-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J. Clin. Invest. 91, 2320–2325 (1993).
Ruhl, S. & Broz, P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ efflux. Eur. J. Immunol. 45, 2927–2936 (2015).
Hardt, W.D., Chen, L.M., Schuebel, K.E., Bustelo, X.R. & Galán, J. E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93, 815–826 (1998).
Acknowledgements
Work in the M.R. laboratory is supported by Public Health Service Grants AI083663, AI101784, AI105374, AI114625, and DK058057. M.R. holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund. A.P.L. was funded by a UC MEXUS-CONACYT award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Commensal microorganisms
-
Bacteria, fungi or viruses that inhabit the host without causing harm.
- Pathobionts
-
Commensal organisms that, under certain circumstances, can cause disease.
- Enteric pathogens
-
Pathogens that cause disease in the intestines.
- Colonization resistance
-
The process by which commensal organisms protect against infection with exogenous microorganisms.
- Siderophore
-
A low molecular weight, high-affinity iron-binding molecule that is secreted by bacteria and fungi to acquire iron from the surrounding environment.
Rights and permissions
About this article
Cite this article
Perez-Lopez, A., Behnsen, J., Nuccio, SP. et al. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol 16, 135–148 (2016). https://doi.org/10.1038/nri.2015.17
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2015.17
This article is cited by
-
Microbiome modulation in inflammatory diseases: Progress to microbiome genetic engineering
Cancer Cell International (2023)
-
Helicobacter pylori infection and small intestinal bacterial overgrowth: a systematic review and meta-analysis
BMC Microbiology (2023)
-
Effect of probiotics as an immune modulator for the management of COVID-19
Archives of Microbiology (2023)
-
QseC sensor kinase modulates the human microbiota during enterohemorrhagic Escherichia coli O157:H7 infection in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®)
Brazilian Journal of Microbiology (2023)
-
An updated review on oral protein-based antigen vaccines efficiency and delivery approaches: a special attention to infectious diseases
Archives of Microbiology (2023)