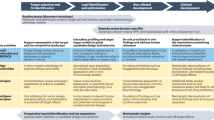
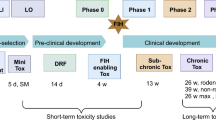
Abstract
Although the principles of preclinical safety evaluation are similar between conventional pharmaceuticals and biotechnology-derived pharmaceuticals (biotech products), the difference lies in the way that these principles are put into practice. The biggest challenge in the preclinical assessment of biotech products has been coping with species specificity and the associated detection and implications of altered immune status. The choice of animal model and study design should depend on the question being asked. This article explores to what extent animal toxicity studies can lead to safer drugs in humans?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zbinden, G. Biotechnology products intended for human use, toxicological targets and research strategies. Prog. Clin. Biol. Res. 235, 143–159 (1987).
Claude, J. R. Difficulties in conceiving and applying guidelines for the safety evaluation of biotechnologically produced drugs: some examples. Toxicol. Lett. 64–65, 349–355 (1992).
Cavagnaro, J. A. Science-based approach to preclinical safety evaluation of biotechnology products. Pharm. Eng. 12, 32–33 (1992).
Dayan, A. D. Safety evaluation of biological and biotechnology-derived medicines. Toxicology 105, 59–68 (1995).
Maki, E. Safety assessment of biotechnology-derived pharmaceutical products. General principles and the relevant cases. J. Toxicol. Sci. 21, 531–534 (1996).
Serabian, M. A. & Pilaro, A. M. Safety assessment of biotechnology-derived pharmaceuticals: ICH and beyond. Toxicol. Pathol. 27, 27–31 (1999).
Bass, R., Purves, J. & Amati, M. P. Safety of biotechnological products. Pharmacol. Toxicol. 86 (Suppl. 1), 27–96 (2000).
Cavagnaro, J. A. in Comprehensive Toxicology 2. Toxicity Testing & Evaluation (eds Williams, P. D. & Hottendorf, G. H.) 291–298 (Elsevier Science, New York, 1996).
Griffiths, S. A. & Lumley, C. (eds) Proceedings of the CMR International Workshop on Safety Evaluation of Biotechnology-Derived Pharmaceuticals: Facilitating a Scientific Approach (CMR International, Surrey, UK, 1997).
Black, L. E. et al. Safety evaluation of immunomodulatory biopharmaceuticals: can we improve the predictive value of preclinical studies? Hum. Exp. Toxicol. 19 (Special issue), 1–265 (2000).
Sims, J. Assessment of biotechnology products for therapeutic use. Toxicol. Lett. 120, 59–66 (2001).
Federal Register 61, FR2733 (29 January 1996).
Guidance for industry on content and format of investigational new drug applications (INDs) for Phase I studies of drugs, including well-characterized, therapeutic, biotechnology-derived products. Center for Biologics Evaluation and Research [online] (cited 29 April 2002) 〈http://www.fda.gov/cber/gdlns/ind1.pdf〉 (1995).
Guidance for industry Q&A content and format of INDs for Phase I studies of drugs, including well-characterized, therapeutic, biotechnology-derived products. Center for Biologics Evaluation and Research [online] (cited 29 April 2002) 〈http://www.fda.gov/cber/gdlns/qaind1.htm〉 (2000).
Guidance for industry INDs for Phase II and III studies of drugs, including specified therapeutic biotechnology-derived products chemistry, manufacturing, and controls content and format. Center for Biologics Evaluation and Research [online] (cited 29 April 2002) 〈http://www.fda.gov/cber/gdlns/indbiodft.pdf〉 (1999).
ICH guidance: Q6B specifications test procedures and acceptance criteria for biotechnological/biological products. Center for Biologics Evaluation and Research [online] (cited 29 April 2002) 〈http://www.fda.gov/cber/guidelines.htm〉 (1999).
Center for Biologics Evaluation and Review (CBER) product approval information. US Food and Drug Adminisitration [online] (cited 29 April 2002) 〈www.fda.gov/cber/efoi/approve.htm〉 (2002).
Biotechnology industry statistics. Biotechnology Industry Organization [online] (cited 25 April 2002) 〈www.bio.org/er/statistics.asp〉 (2001).
Debouck, C. & Metcalf, B. The impact of genomics on drug discovery. Annu. Rev. Pharmacol. Toxicol. 40, 193–207 (2000).
Harris, S. & Ford, S. M. Transgenic gene knock-outs: functional genomics and therapeutic target selection. Pharmacogenomics 1, 433–443 (2000).
Nuwaysir, E. F., Bittner, M., Trent, J., Barrett, J. C. & Afshari, C. A. Microarrays and toxicology: the advent of toxicogenomics. Mol. Carcinog. 24, 153–159 (1999).
Lovett, R. A. Toxicogenomics. Toxicologists brace for the genomics revolution. Science 289, 536–537 (2000).
Burchiel, S. W. et al. Analysis of genetic and epigenetic mechanisms of toxicity: potential role of toxicogenomics and proteomics in toxicology. Toxicol Sci. 59, 193–195 (2001).
Cunningham, M. J. Genomics and proteomics: the new millennium of drug discovery and development. J. Pharmacol. Toxicol. Methods 44, 291–300 (2000).
Fielden, M. R. & Zacharewski, T. R. Challenges and limitations of gene expression profiling in mechanistic and predictive toxicology. Toxicol. Sci. 60, 6–10 (2001).
Nicholson, J. K., Lindon, J. C. & Holmes, E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 11, 1181–1189 (1999).
Searls, D. B. Using bioinformatics in gene and drug discovery. Drug Discov. Today 5, 135–143 (2000).
Maggio, E. T. & Ramnarayan, K. Recent developments in computational proteomics. Drug Discov. Today 6, 996–1004 (2001).
Loo, J. A., DeJohn, D. E., Du, P., Stevenson, T. I. & Ogorzalek Loo, R. R. Application of mass spectrometry for target identification and characterization. Med. Res. Rev. 19, 307–319 (1999).
Roberts, S. A. High-throughput screening approaches for investigating drug metabolism and pharmacokinetics. Xenobiotica 31, 557–589 (2001).
MacGregor, J. T. et al. New molecular endpoints and methods for routing toxicity testing. Fundam. Appl. Toxicol. 26, 156–173 (1995).
Kensler, T. W., Davidson, N. E., Groopman, J. D. & Munoz, A. Biomarkers and surrogacy: relevance to chemoprevention. IARC Sci. Publ. 154, 27–47 (2001).
ICH guidance: S6 safety evaluation of biotechnology-derived pharmaceuticals. Center for Biologics Evaluation and Research [online] (cited 29 April 2002) 〈http://www.fda.gov/cber/guidelines.htm〉 (1997).
Coscarella, A. et al. Pharmacokinetic and immunogenic behavior of three recombinant human GM-CSF–EPO hybrid proteins in cynomolgus monkeys. Mol. Biotechnol. 10, 115–122 (1998).
Braun, A., Kwee, L., Labow, M. A. & Alsenz, J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon-α (IFN-α) in normal and transgenic mice. Pharm. Res. 14, 1472–1478 (1997).
Wang, D. S. et al. Effect of dosing schedule on pharmacokinetics of α-interferon and anti-α interferon neutralizing antibody in mice. Antimicrob. Agents Chemother. 45, 176–180 (2001).
Von Wussow, P. et al. Immunogenicity of different types of interferons in the treatment of hairy-cell leukemias. N. Engl. J. Med. 319, 1226–1227 (1988).
Miller, L. L. et al. Abrogation of the hematological and biological activities of the interleukin 3/granulocyte– macrophage colony-stimulating factor fusion protein PIXY321 by neutralizing anti-PIXY321 antibodies in cancer patients receiving high-dose carboplatin. Blood 93, 3250–3258 (1999).
Gunn, H. Immunogenicity of recombinant human interleukin-3. Clin. Immunol. Immunopathol. 83, 1–4 (1997).
Finkelman, F. D. et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine–anti-cytokine antibody complexes. J. Immunol. 151, 1235–1244 (1993).
May, L. T. et al. Antibodies chaperone circulating IL-6. Paradoxical effects of anti-IL-6 'neutralizing' antibodies in vivo. J. Immunol. 151, 3225–3236 (1993).
Jones, A. T. & Ziltener, H. J. Enhancement of the biologic effects of interleukin-3 in vivo by anti-interleukin-3 antibodies. Blood 82, 1133–1141 (1993).
Brown, R. A. et al. Enhanced firbroblast conctraction of 3D collagen lattices and integrin expression by TGF-β1 and -β3: mechanoregulatory growth factors? Exp. Cell Res. 274, 310–322 (2002).
Green, J. D. & Black, L. E. Status of preclinical safety assessment of immunomodulatory biopharmaceuticals. Hum. Exp. Toxicol. 19, 208–212 (2000).
Pullai, O., Dhanikaul, A. B. & Panchagnula, R. Drug delivery: an odyssey of 100 years. Curr. Opin. Chem. Biol. 5, 439–446 (2001).
Watts, T. L. & Fasano, A. Modulation of intestinal permeability: a novel and innovative approach for the oral delivery of drugs, macromolecules and antigens. Biotechnol. Genet. Eng. Rev. 17, 433–453 (2001).
FDA guidance concerning demonstration of comparability of human biological products, including therapeutic biotechnology-derived products. Center for Biologics Evaluation and Research [online] (cited 29 April 2002) 〈http://www.fda.gov/cber/gdlns/comptest.txt〉 (1996).
Committee for Proprietary Medicinal Products. Note for guidance on comparability of medicinal products containing biotechnology-derived products as drug substance. The European Agency for the Evaluation of Medicinal Products [online] (cited 29 Apr 02) 〈http://www.emea.eu.int/pdfs/human/bwp/320700en.pdf〉 (2001).
Mordenti, J., Cavagnaro, J. A. & Green, J. D. Design of biological equivalence programs for therapeutic biotechnology products in clinical development: a perspective. Pharm. Res. 13, 1427–1437 (1996).
Schaffner, G., Haase, M. & Giess, S. Criteria for investigation of the product equivalence of monoclonal antibodies for therapeutic and in vivo diagnostic use in case of introduction of changes in the manufacturing process. Biologicals 23, 253–259 (1995).
Polastro, E. T. & Little, A. D. The future of biogenerics. Contract Pharma [online] (cited 29 April 2002) 〈http://www.contractpharma.com/Oct013.htm〉 (2001).
Schellekens, H. & Ryff, J.-C. Biogenerics: the off-patent biotech products. Trends Pharmacol. Sci. 23, 119–121 (2002).
Cavagnaro, J. Preclinical development strategies for new medicines: are the correct questions being asked? Reg. Affairs J. 12, 3–4 (2000).
Schwetz, B. A. Toxicology at the Food and Drug Administration: new century, new challenges. Int. J. Toxicol. 20, 3–8 (2001).
Smith, L. L. Key challenges for toxicologists in the 21st century. Trends Pharmacol. Sci. 22, 281–285 (2001).
ICH guidance: M3 non-clinical safety studies for the conduct of human clinical trials for pharmaceuticals. Center for Biologics Evaluation and Research [online] (cited 29 April 2002) 〈http://www.fda.gov/cber/guidelines.htm〉 (2002).
Cavagnaro, J. & Spindler, P. Methods for predicting tumorigenicity of immunomodulatory biopharmaceuticals. Hum. Exp. Toxicol. 19, 213–215 (2000).
Calabrese, E. J. Suitability of animal models for predictive toxicology: theoretical and practical considerations. Drug Metab. Rev. 15, 505–523 (1984).
Russel, R. W. Essential role of animal models in understanding human toxicities. Neurosci. Biobehav. Rev. 15, 7–11 (1991).
Miller, G. S. New concepts of preparation and use of animal models in toxicology. Reg. Toxicol. Pharmacol. 7, 414–416 (1987).
Dayan, A. Forward-safety evaluation of immunomodulatory biopharmaceuticals: can we improve the predictive value of preclinical studies? Hum. Exp. Toxicol. 19, 206–207 (2000).
Sharma, A. et al. Comparative pharmacodynamics of keliximab and clenoliximab in transgenic mice bearing human CD4. J. Pharmacol. Exp. Ther. 293, 33–41 (2000).
Nishijima, I. et al. A human GM-CSF receptor expressed in transgenic mice stimulates proliferation and differentiation of hemopoietic progenitors to all lineages in response to human GM-CSF. Mol. Biol. Cell 6, 497–508 (1995).
Terrell, T. G. & Green, J. D. Comparative pathology of recombinant murine interferon-γ in mice and recombinant human interferon-γ in cynomolgus monkeys. Int. Rev. Exp. Pathol. 34, 73–101 (1993).
Anderson, T. D. & Hayes, T. J. Toxicity of human recombinant interleukin-2 in rats. Pathologic changes are characterized by marked lymphocytic and eosinophilic proliferation and multisystem involvement. Lab. Invest. 60, 331–346 (1989).
Anderson, T. D., Areco, R. & Hayes, T. J. Comparable toxicology and pathology associated with administration of recombinant HuIL-1α to animals. Int. Rev. Exp. Pathol. 34, 9–36 (1993).
Acknowledgements
The author acknowledges fellow members of the ICH S6 'relevant' Expert Working Group — G. Vicari, J. Sims, J. Carstensen, W. Neumann, T. Inoue, M. Nakadate, E. Maki, M. Kawai and J. Green, and colleagues C. Klingbeil, J. Stoudemire, M. Serabian, A. Pilaro, M. Papaluca-Amati, P. Spindler, D. Kornbrust, P. Smith, T. Hayes, T. Anderson, R. Tyler and others for staying on “Dr Zbinden's track”.
Author information
Authors and Affiliations
Related links
Rights and permissions
About this article
Cite this article
Cavagnaro, J. Preclinical safety evaluation of biotechnology-derived pharmaceuticals. Nat Rev Drug Discov 1, 469–475 (2002). https://doi.org/10.1038/nrd822
Issue Date:
DOI: https://doi.org/10.1038/nrd822
This article is cited by
-
Are innovation and new technologies in precision medicine paving a new era in patients centric care?
Journal of Translational Medicine (2019)
-
Applicability of predictive toxicology methods for monoclonal antibody therapeutics: status Quo and scope
Archives of Toxicology (2017)
-
Role of computer-aided drug design in modern drug discovery
Archives of Pharmacal Research (2015)
-
Regulatorischer Rahmen für neuartige Therapien
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2011)