Key Points
-
Although drugs that lead to cholesterol and lipid lowering have proved to have significant effects in lowering cardiovascular morbidity and mortality, coronary artery disease remains a principal cause of death worldwide. So, there is a clear need for the discovery of additional therapeutic approaches to control this disease adequately. In addition to therapies that target novel mechanisms of regulating lipid metabolism, recent data indicate that targeting the inflammatory process in the developing vascular plaques could be beneficial.
-
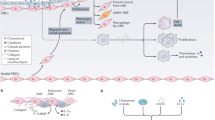
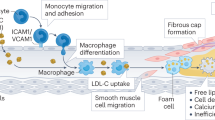
The recruitment, activation and differentiation of monocytes/macrophages to the sub-endothelial space of the nascent vascular lesion, and their subsequent differentiation to lipid-laden foam cells, is the key inflammatory pathway that leads to the development of advanced, necrotic, unstable plaques. Mouse genetics has been used to evaluate the importance of various pathways that are used in the transport of monocytes and macrophages to vascular lesions.
-
A key step in this process is the secretion of inflammatory cytokines, chemoattractants and other reactive molecules from the vascular endothelium and underlying smooth muscle layer after activation by lipids or lipoproteins. These inflammatory molecules stimulate the endothelium to synthesize adhesion molecules, which results in the selective attraction of activated platelets and monocytes to the activated endothelial surface.
-
Mouse studies indicate that low-affinity interactions of selectins and integrins with their cognate ligands might mediate the initial attraction of these circulating vascular cells to the endothelial layer. These low-affinity interactions lead to a slowing, or rolling, of the cells on the endothelium. High-affinity interactions with activated integrins lead to arrest, or firm adherence, of the cells to the endothelial surface. Current data indicate that the E- and P-selectins, as well as vascular cell adhesion molecule 1 (VCAM-1) and the α4β1-integrin (also known as very late (activation) antigen 4; VLA-4), are responsible for mediating rolling and firm arrest of leukocytes on the endothelial surface. After arrest, monocytes migrate across the endothelium in response to a gradient of chemoattractant. The chemokine MCP-1, through its interaction with the monocyte chemokine (CC) motif receptor 2 (CCR2), seems to have an important role in this process. These data indicate that the antagonism of one or more of these monocyte-transport pathways could be of therapeutic use in atherosclerosis.
-
After recruitment of the monocyte/macrophage to the nascent vascular lesion, these cells differentiate into lipid-laden foam cells. The accumulation of lipoprotein particles is mediated by macrophage scavenger receptors, such as MSR-A. After endocytosis of the lipoprotein particles, free cholesterol is released into the cytoplasm, where it can activate the nuclear receptor LXR, which regulates the expression of several key proteins, including the cholesterol efflux pump ABCA1 (ATP-binding cassette, subfamily A, member 1) and the anti-atherogenic apolipoprotein E. A key regulator of this process is the cholesterol esterification–hydrolysis cycle, which modulates the cytoplasmic levels of free cholesterol. Acyl-coenzyme A cholesterol-acetyltransferase (ACAT) esterifies free cholesterol derived from the diet or from lysosomal hydrolysis of endocytosed lipoproteins, and cytoplasmic neutral cholesterol esterases complete the cycle. ACAT inhibitors, such as Pfizer's Avasimibe, are now in advanced clinical trials for the treatment of coronary artery disease.
-
The macrophage/foam cell actively secretes inflammatory cytokines and other reactive molecules that escalate the inflammatory response and ultimately lead to the development of necrotic lesions that are covered with an unstable fibrous cap and are susceptible to rupture. Macrophage metalloproteinases are important mediators of plaque rupture, as well as the arterial remodelling process that is associated with plaque development.
-
Therapies targeted to the attenuation of cellular recruitment, or to the modification of macrophage/foam cell differentiation, should prove to be useful in the treatment of coronary artery disease.
Abstract
Although drugs that lead to cholesterol and lipid lowering have proved to have significant effects in lowering cardiovascular morbidity and mortality, coronary artery disease remains a principal cause of death worldwide. There is a clear need to discover further therapeutic approaches to control this disease adequately. This review focuses on the mechanisms that have been implicated in the recruitment, activation and differentiation of inflammatory monocytes/macrophages in nascent vascular lesions into lipid-laden foam cells. These mechanisms might provide attractive targets for novel therapies for coronary artery disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Meredith, I. T. et al. Role of endothelium in ischemic coronary syndromes. Am. J. Cardiol. 72, 27C–31C; discussion, 31C–32C (1993).
Cannon, R. O. Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin. Chem. 44, 1809–1819 (1998).
Kauser, K., da Cunha, V., Fitch, R., Mallari, C. & Rubanyi, G. M. Role of endogenous nitric oxide in progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 278, H1679–H1685 (2000).
Springer, T. A. & Cybulsky, M. I. in Atherosclerosis and Coronary Artery Disease Vol. 1 (eds Fuster, V., Ross, R. & Topol, E. J.) 511–538 (Lippincott-Raven, Philadelphia, 1996).
Stary, H. C. et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis: a report from The Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 89, 2462–2478 (1994).
Falk, E., Shah, P. K. & Fuster, V. in Atherosclerosis and Coronary Artery Disease Vol. 1 (eds Fuster, V., Ross, R. & Topol, E. J.) 492–510 (Lippincott-Raven, Philadelphia, 1996).
Schoenhagen, P., Ziada, K. M., Vince, D. G., Nissen, S. E. & Tuzco, E. M. Arterial remodeling and coronary artery disease: the concept of 'dilated' versus 'obstructive' coronary atherosclerosis. J. Am. Coll. Cardiol. 38, 297–306 (2001).This paper describes an important new concept in the diagnosis of coronary artery disease, and has implications for the evaluation of therapies that target vascular-wall pathology.
Faggiotto, A. & Ross, R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis 4, 341–356 (1984).
Bocan, T. M. Animal models of atherosclerosis and interpretation of drug intervention studies. Curr. Pharm. Des. 4, 37–52 (1998).
Paigen, B., Morrow, A., Brandon, C., Mitchell, D. & Williams, R. A. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 57, 65–73 (1985).
Nakashima, Y., Plump, A. S., Raines, E. W., Breslow, J. L. & Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 14, 133–140 (1994).
Johnson, J. L. & Jackson, C. L. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis 154, 399–406 (2001).
Ishibashi, S. et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92, 883–893 (1993).
Hirano, K. et al. Targeted disruption of the mouse Apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. J. Biol. Chem. 271, 9887–9890 (1996).
Powell-Braxton, L. et al. A mouse model for familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nature Med. 4, 934–938 (1998).
Sanan, D. A. et al. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). Proc. Natl Acad. Sci. USA 95, 4544–4549 (1998).References 16 and 17 , along with reference 12 , are key descriptions of the most useful mouse models of atherosclerosis.
Veniant, M. M., Withycombe, S. & Soung, S. G. Lipoprotein size and atherosclerosis susceptibility in Apoe−/− and Ldlr− mice. Arterioscler. Thromb. Vasc. Biol. 21, 567–570 (2001).
Dong, Z. M. et al. The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 102, 145–152 (1998).These data show the importance of the selectins in modulating cell transport to the vascular endothelium.
Ramos, C. L. et al. Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ. Res. 84, 1237–1244 (1999).
Dong, Z. M., Brown, A. A. & Wagner, D. D. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation 101, 2290–2295 (2000).
Iiyama, K. et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ. Res. 85, 199–207 (1999).
Bourdillon, M. C. et al. ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE−/−/CAM-1−/− fed a fat or a chow diet. Arterioscler. Thromb. Vasc. Biol. 20, 2630–2635 (2000).
Cybulsky, M. I. et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 107, 1255–1262 (2001).Differentiates VCAM as being a key molecule for mediation of monocyte adhesion in the vascular endothelium.
Shih, P. T. et al. Blocking very late antigen-4 integrin decreases leukocyte entry and fatty streak formation in mice fed an atherogenic diet. Circ. Res. 84, 345–351 (1999).
Cascieri, M. A. & Springer, M. S. The chemokine/chemokine receptor family: potential and progress for therapeutic intervention. Curr. Opin. Chem. Biol. 4, 420–427 (2000).
Yla-Herttuala, S. et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl Acad. Sci. USA 88, 5252–5256 (1991).
Gosling, J. et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J. Clin. Invest. 103, 773–778 (1999).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).Implicates the chemokine receptor CCR2 in the development of vascular lesions in mice.
Szalai, C. et al. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1 −2518 G/G genotype in CAD patients. Atherosclerosis 158, 233–239 (2001).Reference 30 provides preliminary human genetic data that show the relationship between the chemokine ligand for the CCR2 receptor and coronary artery disease, highlighting the power of using human genetics to assess various mechanisms identified in mouse models.
Moatti, D. et al. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood 97, 1925–1928 (2001).
Cyrus, T. et al. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in ApoE-deficient mice. J. Clin. Invest. 103, 1597–1604 (1999).
De Winther, M. P. & Hofker, M. H. Scavenging new insights into atherogenesis. J. Clin. Invest. 105, 1039–1041 (2000).
Suzuki, H. et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–296 (1997).
Nicholson, A. C., Febbraio, M., Han, J., Silverstein, R. L. & Hajjar, D. P. CD36 in atherosclerosis. The role of a class B macrophage scavenger receptor. Ann. NY Acad. Sci. 902, 128–131; discussion 131–133 (2000). | PubMed |
Brewer, H. B. The lipid-laden foam cell: an elusive target for therapeutic intervention. J Clin Invest 105, 703–705 (2000).
Krause, R. R. & Bocan, T. M. A. in Inflammation, Mediators and Pathways (eds Ruffolo, R. R. Jr & Hollinger, M. A.) 173–198 (CRC, Florida, 1995).
Peet, D. J. et al. Cholesterol and bile acid metabolism are impaired in mice lacking nuclear oxysterol receptor LXRα. Cell 93, 693–704 (1998).
Alberti, S. et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRβ-deficient mice. J. Clin. Invest. 107, 565–573 (2001).
Venkateswaran, A. et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc. Natl Acad. Sci. USA 97, 12097–12102 (2000).
Laffitte, B. A. et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl Acad. Sci. USA 98, 507–512 (2001).References 40 and 41 provide data indicating that the lipid receptor LXR might have an important role in the development of the vascular-wall macrophage/foam cell, in addition to its role in modulating hepatic cholesterol metabolism.
Makowski, L. et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nature Med. 7, 699–705 (2001).
Hofmann, M. A. et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J. Clin. Invest. 107, 675–683 (2001).
Brand, K. et al. Activated transcription factor nuclear factor-κB is present in the athersclerotic lesion. J. Clin. Invest. 97, 1715–1722 (1996).
Denk, A. et al. Activation of NF-κB via the IκB kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J. Biol. Chem. 276, 28451–28458 (2001).
Li, Y., Chi, L., Stechschulte, D. J. & Dileepan, K. N. Histamine-induced production of interleukin-6 and interleukin-8 by human coronary artery endothelial cells is enhanced by endotoxin and tumor necrosis factor-κ. Microvasc. Res. 61, 253–262 (2001).
Dichtl, W. et al. Very low-density lipoprotein activates nuclear factor-κB in endothelial cells. Circ. Res. 84, 1085–1094 (1999).
Staels, B. et al. Activation of human aortic smooth-muscle cells is inhibitied by PPARγ but not by PPARγ activators. Nature 393, 790–793 (1998).
Detmers, P. A. et al. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 165, 3430–3435 (2000).
Huber, S. A., Sakkinen, P., Conze, D., Hardin, N. & Tracy, R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 19, 2364–2367 (1999).
Sukovich, D. A. et al. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17β-estradiol. Arterioscler. Thromb. Vasc. Biol. 18, 1498–1505 (1998).
Albert, M. A. & Ridker, P. M. The role of C-reactive protein in cardiovascular disease risk. Curr. Cardiol. Rep. 1, 99–104 (1999).
Ridker, P. M. et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 344, 1959–1965 (2001).
Sparrow, C. P. et al. Simvastatin has anti-inflammatory and anti-atherosclerotic activities independent of plasma cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 21, 115–121 (2001).
Melian, A., Geng, Y. J., Sukhova, G. K., Libby, P. & Porcelli, S. A. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am. J. Pathol. 155, 775–786 (1999).
Gupta, S. et al. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, 2752–2761 (1997).
Dansky, H. M., Charlton, S. A., Harper, M. M. & Smith, J. D. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc. Natl Acad. Sci. USA 94, 4642–4646 (1997).
Daugherty, A. et al. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E−/− mice. J. Clin. Invest. 100, 1575–1580 (1997).
Song, L., Leung, C. & Schindler, C. Lymphocytes are important in early atherosclerosis. J. Clin. Invest. 108, 251–259 (2001).
Nicoletti, A., Paulsson, G., Caligiuri, G., Zhou, X. & Hansson, G. K. Induction of neonatal tolerance to oxidized lipoprotein reduces atherosclerosis in ApoE knockout mice. Mol. Med. 6, 283–290 (2000).
Teo, K. K. et al. Long-term effects of cholesterol lowering and angiotensin-converting enzyme inhibition on coronary atherosclerosis: the Simvastatin/Enalapril Coronary Atherosclerosis Trial (SCAT). Circulation 102, 1748–1754 (2000).
Von Birgelen, C. et al. Plaque distribution and vascular remodeling of ruptured and nonruptured coronary plaques in the same vessel: an intravascular ultrasound study in vivo. J. Am. Coll. Cardiol. 37, 1864–1870 (2001).
Takano, M. et al. Mechanical and structural characteristics of vulnerable plaques: analysis by coronary angioscopy and intravascular ultrasound. J. Am. Coll. Cardiol. 38, 99–104 (2001).
Kaneko, E. et al. Detection of dissection and remodeling of atherosclerotic lesions in rabbits after balloon angioplasty by magnetic-resonance imaging. Coron. Artery Dis. 11, 599–606 (2000).
Fischer, A., Gutstein, D. E., Fayad, Z. A. & Fuster, V. Predicting plaque rupture: enhancing diagnosis and clinical decision-making in coronary artery disease. Vasc. Med. 5, 163–172 (2000).
Coombs, B. D., Rapp, J. H., Ursell, P. C., Reilly, L. M. & Saloner, D. Structure of plaque at carotid bifurcation: high-resolution MRI with histological correlation. Stroke 32, 2516–2521 (2001).
Zhao, X. Q. et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler. Thromb. Vasc. Biol. 21, 1623–1629 (2001).
Hoffmann, U. et al. Quantification of coronary artery calcification in patients with FH using EBCT. Eur. J. Clin. Invest. 31, 471–475 (2001).
Chen, L. C. et al. Differential coronary artery calcification detected by electron beam computed tomography as an indicator of coronary stenosis among patients with stable angina pectoris. Can. J. Cardiol. 17, 667–676 (2001).
Acknowledgements
M.A.C. would like to acknowledge helpful discussions with S. Wright, Merck Research Laboratiries, during the preparation of this review.
Author information
Authors and Affiliations
Related links
Glossary
- HYPERLIPIDAEMIA
-
An excess of lipids, either triglycerides or cholesterol, in the blood. Lipids circulate as free or esterified entities in lipoprotein particles.
- ATHEROSCLEROTIC PLAQUE
-
A lesion within the intima and media of large- and medium-sized arteries that contains high levels of lipids, lipoproteins, activated macrophages, lipid-enriched foam cells and smooth muscle cells. Advanced lesions can become necrotic, and are covered with a fibrous cap that can rupture and cause catastrophic blockage of coronary arteries.
- LOW-DENSITY LIPOPROTEIN (LDL).
-
A class of lipoprotein that carries cholesterol through the bloodstream. The surface of an LDL particle is a monolayer of phospholipid and unesterified cholesterol. The core is hydrophobic, and is rich in fatty esters of cholesterol. The hydrophobic apoliprotein B is embedded in the membrane.
- LEUKOCYTE
-
A general term used to describe any lymphoid cell. Includes all nucleated blood cells that do not contain haemoglobin.
- EXTRACELLULAR MATRIX
-
A complex, three-dimensional network of very large macromolecules that provides contextual information and an architectural scaffold for cellular adhesion and migration.
- MYOCARDIAL INFARCTION
-
Popularly known as a heart attack, this is the death of part of the heart muscle due to sudden loss of blood supply. Typically, the loss of this supply is caused by the complete blockage of a coronary artery by a blood clot.
- ANGIOGRAPHY
-
An X-ray of arteries and veins that is used to diagnose blockages. A catheter is inserted into the vessel, and a contrast agent is injected so that the vessels will be visible on an X-ray.
- LOW-DENSITY LIPOPROTEIN RECEPTOR
-
An endocytotic hepatic receptor that binds apolipoprotein B, thereby internalizing low-density lipoprotein (LDL), which leads to processing that regulates cholesterol and LDL synthesis. Genetic defects in LDL receptors lead to abnormal serum levels of LDL and hyperlipidaemia.
- CHOW DIET
-
A mouse diet with a relatively low fat and cholesterol content. A typical chow diet may contain 4.5% fat and 0.02% cholesterol by weight, whereas a typical high-fat diet (or Western diet) may contain 21% fat and 0.15% cholesterol by weight.
- VERY-LOW-DENSITY LIPOPROTEIN
-
A smaller lipoprotein particle than LDL, the protein component of which is apolipoprotein E.
- INTIMA
-
The innermost layer of endothelial cells in arteries and veins.
Rights and permissions
About this article
Cite this article
Cascieri, M. The potential for novel anti-inflammatory therapies for coronary artery disease. Nat Rev Drug Discov 1, 122–130 (2002). https://doi.org/10.1038/nrd723
Issue Date:
DOI: https://doi.org/10.1038/nrd723
This article is cited by
-
„Warum trifft der Herzinfarkt immer das Herz?“
Herz (2010)
-
Macrophage LXRs inhibit atherosclerosis
Nature Reviews Drug Discovery (2002)