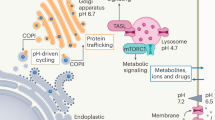
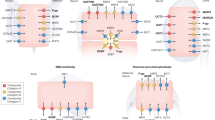
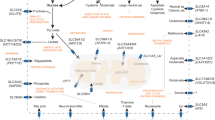
Key Points
-
Membrane transport proteins mediate the transport of molecules across cell membranes and have key roles in human health. More than 100 Mendelian diseases are caused by a defect in a single solute carrier (SLC) transporter.
-
Genetic studies have provided a wealth of information on the roles that SLC transporters play in human health, and in common and rare diseases, enhancing our understanding of the biology of these membrane transporters.
-
High-throughput screening technologies and computational methods may be used to discover novel inhibitors and activators of SLC transporters for therapeutic purposes.
-
Utilizing transporters as drug targets may require indirect methods, such as developing molecules that function as potentiators or correctors, or developing substrates that bypass the transporter.
-
Some currently marketed drugs, including diuretics, neuropsychiatric drugs and antidiabetic drugs, target SLC transporters.
-
Uric acid-, glycine- and bile acid-transport inhibitors are currently in various stages of clinical development for the treatment of various human diseases. First-in-class compounds that target SLC transporters are anticipated to be approved in the near future.
-
Positron emission tomography (PET)-imaging probes may utilize transporters for uptake into cells, enabling transporter function to be visualized in vivo.
Abstract
Solute carrier (SLC) transporters — a family of more than 300 membrane-bound proteins that facilitate the transport of a wide array of substrates across biological membranes — have important roles in physiological processes ranging from the cellular uptake of nutrients to the absorption of drugs and other xenobiotics. Several classes of marketed drugs target well-known SLC transporters, such as neurotransmitter transporters, and human genetic studies have provided powerful insight into the roles of more-recently characterized SLC transporters in both rare and common diseases, indicating a wealth of new therapeutic opportunities. This Review summarizes knowledge on the roles of SLC transporters in human disease, describes strategies to target such transporters, and highlights current and investigational drugs that modulate SLC transporters, as well as promising drug targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Giacomini, K. M. et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215–236 (2010). This white paper by the International Transporter Consortium provides guidance on which transporters may be clinically relevant and the types of in vitro and in vivo studies needed to study drug–transporter interactions.
Ng, S. B. et al. Exome sequencing identifies the cause of a Mendelian disorder. Nat. Genet. 42, 30–35 (2010). This is the first study to use exome sequencing to identify a gene associated with Miller syndrome.
Schlessinger, A., Yee, S. W., Sali, A. & Giacomini, K. M. SLC classification: an update. Clin. Pharmacol. Ther. 94, 19–23 (2013).
Hediger, M. A., Clémençon, B., Burrier, R. E. & Bruford, E. A. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol. Aspects Med. 34, 95–107 (2013). This review provides a comprehensive guide to the different families of SLC transporters.
Geier, E. G. et al. Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc. Natl Acad. Sci. USA 110, 5480–5485 (2013).
Fotiadis, D., Kanai, Y. & Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 34, 139–158 (2013).
Izumi, S. et al. Substrate-dependent inhibition of organic anion transporting polypeptide 1B1: comparative analysis with prototypical probe substrates estradiol-17β-glucuronide, estrone-3-sulfate, and sulfobromophthalein. Drug Metab. Dispos. 41, 1859–1866 (2013).
Hagenbuch, B. & Stieger, B. The SLCO (former SLC21) superfamily of transporters. Mol. Aspects Med. 34, 396–412 (2013).
Koepsell, H. & Endou, H. The SLC22 drug transporter family. Pflugers Arch. 447, 666–676 (2004).
Schlessinger, A. et al. Comparison of human solute carriers. Protein Sci. 19, 412–428 (2010).
Schlessinger, A. et al. Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET. Proc. Natl Acad. Sci. USA 108, 15810–1 5815 (2011). This study uses virtual screening against a comparative model of an SLC transporter to identify prescription drugs that may interact with the transporter. This method is also used for other SLC transporters (for example, see references 5 and 64).
Bröer, S. Apical transporters for neutral amino acids: physiology and pathophysiology. Physiology (Bethesda) 23, 95–103 (2008).
Mueckler, M. & Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 34, 121–138 (2013).
Wright, E. M. Glucose transport families SLC5 and SLC50. Mol. Aspects Med. 34, 183–196 (2013).
Jostins, L. et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Kenny, E. E. et al. A genome-wide scan of Ashkenazi Jewish Crohn's disease suggests novel susceptibility loci. PLoS Genet. 8, e1002559 (2012).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Kottgen, A. et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 45, 145–154 (2013). This is the first GWAS to identify a genetic variant in SLC2A9 that is associated with serum uric acid levels.
Tin, A. et al. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum. Mol. Genet. 20, 4056–4068 (2011).
Li, S. et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 3, e194 (2007).
Dai, X. et al. A genome-wide association study for serum bilirubin levels and gene–environment interaction in a Chinese population. Genet. Epidemiol. 37, 293–300 (2013).
Bielinski, S. J. et al. Mayo Genome Consortia: a genotype–phenotype resource for genome-wide association studies with an application to the analysis of circulating bilirubin levels. Mayo Clin. Proc. 86, 606–614 (2011).
Sanna, S. et al. Common variants in the SLCO1B3 locus are associated with bilirubin levels and unconjugated hyperbilirubinemia. Hum. Mol. Genet. 18, 2711–2718 (2009).
Johnson, A. D. et al. Genome-wide association meta-analysis for total serum bilirubin levels. Hum. Mol. Genet. 18, 2700–2710 (2009).
Nan, H. et al. Genome-wide association study of tanning phenotype in a population of European ancestry. J. Invest. Dermatol. 129, 2250–2257 (2009).
Stokowski, R. P. et al. A genomewide association study of skin pigmentation in a South Asian population. Am. J. Hum. Genet. 81, 1119–1132 (2007).
Dhumeaux, D. & Erlinger, S. Hereditary conjugated hyperbilirubinaemia: 37 years later. J. Hepatol. 58, 388–390 (2013).
Sladek, R. et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445, 881–885 (2007).
Scott, L. J. et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316, 1341–1345 (2007).
Rafnar, T. et al. European genome-wide association study identifies SLC14A1 as a new urinary bladder cancer susceptibility gene. Hum. Mol. Genet. 20, 4268–4281 (2011).
Ehret, G. B. et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011).
Wain, L. V. et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat. Genet. 43, 1005–1011 (2011).
Fletcher, O. et al. Novel breast cancer susceptibility locus at 9q31.2: results of a genome-wide association study. J. Natl Cancer Inst. 103, 425–435 (2011).
Zhang, K. et al. Genetic implication of a novel thiamine transporter in human hypertension. J. Am. Coll. Cardiol. 63, 1542–1555 (2014).
Nicolson, T. J. et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58, 2070–2083 (2009).
Ishihara, H., Maechler, P., Gjinovci, A., Herrera, P.-L. & Wollheim, C. B. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat. Cell Biol. 5, 330–335 (2003).
Kim, B. J. et al. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes 49, 367–372 (2000).
Tamaki, M. et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Invest. 123, 4513–4524 (2013).
Flannick, J. et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 46, 357–363 (2014). This paper demonstrates that loss-of-function variants in SLC30A8 are protective against type 2 diabetes.
Hu, G. et al. New fluorescent substrate enables quantitative and high-throughput examination of vesicular monoamine transporter 2 (VMAT2). ACS Chem. Biol. 8, 1947–1954 (2013).
Ulanovskaya, O. A., Cui, J. & Kron, S. J. & Kozmin, S. A. A pairwise chemical genetic screen identifies new inhibitors of glucose transport. Chem. Biol. 18, 222–230 (2011).
Wittwer, M. B. et al. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J. Med. Chem. 56, 781–795 (2013).
Kido, Y., Matsson, P. & Giacomini, K. M. Profiling of a prescription drug library for potential renal drug–drug interactions mediated by the organic cation transporter 2. J. Med. Chem. 54, 4548–4558 (2011). This is one of the first HTS studies to use fluorescent probes as a transporter substrate to identify prescription drugs that inhibit SLC transporters, and its potential for transporter-mediated drug–drug interactions. This method is also used for other SLC transporters (for example, see references 42 and 44).
Gui, C., Obaidat, A., Chaguturu, R. & Hagenbuch, B. Development of a cell-based high-throughput assay to screen for inhibitors of organic anion transporting polypeptides 1B1 and 1B3. Curr. Chem. Genom. 4, 1–8 (2010).
Jani, M. & Krajcsi, P. In vitro methods in drug transporter interaction assessment. Drug Discov. Today. Technol. 12, e105–e112 (2014).
Pedersen, J. M. et al. Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol. Sci. 136, 328–343 (2013).
Sodani, K. et al. Telatinib reverses chemotherapeutic multidrug resistance mediated by ABCG2 efflux transporter in vitro and in vivo. Biochem. Pharmacol. 89, 52–61 (2014).
Pedersen, J. M. et al. Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2). J. Med. Chem. 51, 3275–3287 (2008).
Zhang, J.-H., Chung, T. & Oldenburg, K. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Thorne, N., Auld, D. S. & Inglese, J. Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr. Opin. Chem. Biol. 14, 315–324 (2010).
Feng, B. Y. et al. A high-throughput screen for aggregation-based inhibition in a large compound library. J. Med. Chem. 50, 2385–2390 (2007).
Jadhav, A. et al. Quantitative analyses of aggregation, autofluorescence, and reactivity artifacts in a screen for inhibitors of a thiol protease. J. Med. Chem. 53, 37–51 (2010).
Caporuscio, F. & Tafi, A. Pharmacophore modelling: a forty year old approach and its modern synergies. Curr. Med. Chem. 18, 2543–2553 (2011).
Cumming, J. G., Davis, A. M., Muresan, S., Haeberlein, M. & Chen, H. Chemical predictive modelling to improve compound quality. Nat. Rev. Drug Discov. 12, 948–962 (2013).
Zheng, X., Ekins, S., Raufman, J. & Polli, J. E. Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol. Pharm. 6, 1591–1603 (2009).
Zheng, X., Pan, Y., Acharya, C., Swaan, P. W. & Polli, J. E. Structural requirements of the ASBT by 3D-QSAR analysis using aminopyridine conjugates of chenodeoxycholic acid. Bioconjug. Chem. 21, 2038–2048 (2010).
Esslinger, C. S. et al. The substituted aspartate analogue l-β-threo-benzyl-aspartate preferentially inhibits the neuronal excitatory amino acid transporter EAAT3. Neuropharmacology 49, 850–861 (2005).
Macdougall, I. J. A. & Griffith, R. Pharmacophore design and database searching for selective monoamine neurotransmitter transporter ligands. J. Mol. Graph. Model. 26, 1113–1124 (2008).
Sharma, H. et al. Flexible and biomimetic analogs of triple uptake inhibitor 4-((((3S,6S)-6-benzhydryltetrahydro-2H-pyran-3-yl)amino)methyl)phenol: synthesis, biological characterization, and development of a pharmacophore model. Bioorg. Med. Chem. 22, 311–324 (2014).
Santra, S. et al. Structural exploration of (3S,6S)-6-benzhydryl-N-benzyltetrahydro-2H-pyran-3-amine analogues: identification of potent triple monoamine reuptake inhibitors as potential antidepressants. ChemMedChem 7, 2093–2100 (2012).
Thompson, C. M. et al. Inhibitor of the glutamate vesicular transporter (VGLUT). Curr. Med. Chem. 12, 2041–2056 (2005).
Ohtake, Y. et al. Discovery of tofogliflozin, a novel C-arylglucoside with an O-spiroketal ring system, as a highly selective sodium glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 55, 7828–7840 (2012).
Schlessinger, A., Khuri, N., Giacomini, K. M. & Sali, A. Molecular modeling and ligand docking for solute carrier (SLC) transporters. Curr. Top. Med. Chem. 13, 843–856 (2013).
Schlessinger, A. et al. High selectivity of the γ-aminobutyric acid transporter 2 (GAT-2, SLC6A13) revealed by structure-based approach. J. Biol. Chem. 287, 37745–37756 (2012).
Luethi, E. et al. Identification of selective norbornane-type aspartate analogue inhibitors of the glutamate transporter 1 (GLT-1) from the chemical universe generated database (GDB). J. Med. Chem. 53, 7236–7250 (2010).
Johnson, Z. L. et al. Structural basis of nucleoside and nucleoside drug selectivity by concentrative nucleoside transporters. eLife 3, e03604 (2014).
Johnson, Z. L., Cheong, C.-G. & Lee, S.-Y. Crystal structure of a concentrative nucleoside transporter from Vibrio cholerae at 2.4 Å. Nature 483, 489–493 (2012).
Zhou, Z. et al. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science 317, 1390–1393 (2007).
Singh, S. K., Yamashita, A. & Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 448, 952–956 (2007).
Penmatsa, A., Wang, K. H. & Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 503, 85–90 (2013).
Cuboni, S. & Hausch, F. Snapshot of antidepressants at work: the structure of neurotransmitter transporter proteins. Angew. Chem. Int. Ed. Engl. 53, 5008–5009 (2014).
Cheah, B. C., Vucic, S., Krishnan, A. V. & Kiernan, M. C. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr. Med. Chem. 17, 1942–1959 (2010).
Fumagalli, E., Funicello, M., Rauen, T., Gobbi, M. & Mennini, T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 578, 171–176 (2008). This study is the first to show riluzole as an activator of SLC transporter function.
Carbone, M., Duty, S. & Rattray, M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem. Int. 60, 31–38 (2012).
Dall'Igna, O. P., Bobermin, L. D., Souza, D. O. & Quincozes-Santos, A. Riluzole increases glutamate uptake by cultured C6 astroglial cells. Int. J. Dev. Neurosci. 31, 482–486 (2013).
Woltjer, R. L. et al. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J. Neuropathol. Exp. Neurol. 69, 667–676 (2010).
Guo, H. et al. Increased expression of the glial glutamate transporter EAAT2 modulates excitotoxicity and delays the onset but not the outcome of ALS in mice. Hum. Mol. Genet. 12, 2519–2532 (2003). This paper describes the function of ivacaftor as a CFTR potentiator.
Kong, Q. et al. Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection. J. Clin. Invest. 124, 1255–1267 (2014). This study demonstrates that an EAAT2 activator can provide neuroprotection in an animal model of amyotrophic lateral sclerosis.
Lin, C.-L. G., Kong, Q., Cuny, G. D. & Glicksman, M. A. Glutamate transporter EAAT2: a new target for the treatment of neurodegenerative diseases. Future Med. Chem. 4, 1689–1700 (2012).
Su, Z. et al. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2). Proc. Natl Acad. Sci. USA 100, 1955–1960 (2003).
Colton, C. K. et al. Identification of translational activators of glial glutamate transporter EAAT2 through cell-based high-throughput screening: an approach to prevent excitotoxicity. J. Biomol. Screen. 15, 653–662 (2010).
Van Goor, F. et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl Acad. Sci. USA 106, 18825–18830 (2009).
Hoffman, L. R. & Ramsey, B. W. Cystic fibrosis therapeutics: the road ahead. Chest 143, 207–213 (2013).
Manzardo, A. M. et al. Double-blind, randomized placebo-controlled clinical trial of benfotiamine for severe alcohol dependence. Drug Alcohol Depend. 133, 562–570 (2013).
Yiu, W. H., Pan, C. J., Allamarvdasht, M., Kim, S. Y. & Chou, J. Y. Glucose-6-phosphate transporter gene therapy corrects metabolic and myeloid abnormalities in glycogen storage disease type Ib mice. Gene Ther. 14, 219–226 (2007).
Hopkins, A. L. & Groom, C. R. The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 (2002).
Rask-Andersen, M., Masuram, S., Fredriksson, R. & Schiöth, H. B. Solute carriers as drug targets: current use, clinical trials and prospective. Mol. Aspects Med. 34, 702–710 (2013).
El-Gebali, S., Bentz, S., Hediger, M. A. & Anderle, P. Solute carriers (SLCs) in cancer. Mol. Aspects Med. 34, 719–734 (2013).
Sophic Alliance. White paper. The integrated druggable genome database. Sophic [online] (2010).
Zhu, F. et al. Therapeutic target database update 2012: a resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 40, D1128–D1136 (2012).
Knox, C. et al. DrugBank 3.0: a comprehensive resource for 'omics' research on drugs. Nucleic Acids Res. 39, D1035–D1041 (2011).
Rask-Andersen, M., Masuram, S. & Schiöth, H. B. The druggable genome: evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu. Rev. Pharmacol. Toxicol. 54, 9–26 (2014).
Wiley, J. S. & Cooper, R. A. A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J. Clin. Invest. 53, 745–755 (1974).
Burg, M., Stoner, L., Cardinal, J. & Green, N. Furosemide effect on isolated perfused tubules. Am. J. Physiol. 225, 119–124 (1973).
Markadieu, N. & Delpire, E. Physiology and pathophysiology of SLC12A1/2 transporters. Pflugers Arch. 466, 91–105 (2014).
Xu, J. C. et al. Molecular cloning and functional expression of the bumetanide-sensitive Na–K–Cl cotransporter. Proc. Natl Acad. Sci. USA 91, 2201–2205 (1994).
Simon, D. B. et al. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na–K–2Cl cotransporter NKCC2. Nat. Genet. 13, 183–188 (1996). This study uses linkage analysis to determine that mutations in SLC12A1 cause Bartter syndrome.
Simon, D. B. et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na–Cl cotransporter. Nat. Genet. 12, 24–30 (1996).
Cha, S. H. et al. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol. Pharmacol. 59, 1277–1286 (2001).
Jutabha, P. et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J. Biol. Chem. 285, 35123–35132 (2010).
Kristensen, A. S. et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 (2011).
Haase, J. & Brown, E. Integrating the monoamine, neurotrophin and cytokine hypotheses of depression — a central role for the serotonin transporter? Pharmacol. Ther. 147, 1–11 (2015).
Wong, D. T., Horng, J. S., Bymaster, F. P., Hauser, K. L. & Molloy, B. B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 15, 471–479 (1974).
Wong, D. T., Bymaster, F. P., Horng, J. S. & Molloy, B. B. A new selective inhibitor for uptake of serotonin into synaptosomes of rat brain: 3-(p-trifluoromethylphenoxy). N-methyl-3- phenylpropylamine. J. Pharmacol. Exp. Ther. 193, 804–811 (1975). This is the first study to demonstrate the ability of fluoxetine to inhibit serotonin uptake in rat synaptosomes.
Jankovic, J. & Clarence-Smith, K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev. Neurother. 11, 1509–1523 (2011).
Nickell, J. R., Siripurapu, K. B., Vartak, A., Crooks, P. A. & Dwoskin, L. P. The vesicular monoamine transporter-2: an important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse. Adv. Pharmacol. 69, 71–106 (2014).
Shibazaki, T. et al. KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J. Pharmacol. Exp. Ther. 342, 288–296 (2012).
Katsuno, K. et al. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J. Pharmacol. Exp. Ther. 320, 323–330 (2007).
Fujimori, Y. et al. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. J. Pharmacol. Exp. Ther. 327, 268–276 (2008).
Fujimori, Y. et al. Sergliflozin etabonate, a selective SGLT2 inhibitor, improves glycemic control in streptozotocin-induced diabetic rats and Zucker fatty rats. Eur. J. Pharmacol. 609, 148–154 (2009).
Ferrannini, E. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124, 499–508 (2014). This paper shows that patients with type 2 diabetes who are treated with SGLT2 inhibitors have improved β-cell function and insulin sensitivity.
Plosker, G. L. Canagliflozin: a review of its use in patients with type 2 diabetes mellitus. Drugs 74, 807–824 (2014).
Forst, T. et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes. Metab. 16, 467–477 (2014).
Cefalu, W. T. et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 382, 941–950 (2013).
Lapuerta, P. et al. Study design and rationale of a dose-ranging trial of LX4211, a dual inhibitor of SGLT1 and SGLT2, in type 2 diabetes inadequately controlled on metformin monotherapy. Clin. Cardiol. 36, 367–371 (2013).
Oliva, R. V. & Bakris, G. L. Blood pressure effects of sodium–glucose co-transport 2 (SGLT2) inhibitors. J. Am. Soc. Hypertens. 8, 330–339 (2014).
George, R. L. & Keenan, R. T. Genetics of hyperuricemia and gout: implications for the present and future. Curr. Rheumatol. Rep. 15, 309 (2013).
Terkeltaub, R. Update on gout: new therapeutic strategies and options. Nat. Rev. Rheumatol. 6, 30–38 (2010).
Anzai, N. & Endou, H. Urate transporters: an evolving field. Semin. Nephrol. 31, 400–409 (2011).
Crittenden, D. B. & Pillinger, M. H. New therapies for gout. Annu. Rev. Med. 64, 325–337 (2013).
Harvey, R. J. & Yee, B. K. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat. Rev. Drug Discov. 12, 866–885 (2013).
Goff, D. C. Bitopertin: the good news and bad news. JAMA Psychiatry 71, 621–622 (2014).
Vandenberg, R. J., Ryan, R. M., Carland, J. E., Imlach, W. L. & Christie, M. J. Glycine transport inhibitors for the treatment of pain. Trends Pharmacol. Sci. 35, 423–430 (2014).
Claro da Silva, T., Polli, J. E. & Swaan, P. W. The solute carrier family 10 (SLC10): beyond bile acid transport. Mol. Aspects Med. 34, 252–269 (2013).
Staels, B. & Fonseca, V. A. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care 32, S237–S245 (2009).
Dawson, P. A. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb. Exp. Pharmacol. 201, 169–203 (2011).
Sakamoto, S. et al. Glucuronidation converting methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-naphthoate (S-8921) to a potent apical sodium-dependent bile acid transporter inhibitor, resulting in a hypocholesterolemic action. J. Pharmacol. Exp. Ther. 322, 610–618 (2007).
Oelkers, P., Kirby, L. C., Heubi, J. E. & Dawson, P. A. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J. Clin. Invest. 99, 1880–1887 (1997).
Lundasen, T. et al. Inhibition of intestinal bile acid transporter Slc10a2 improves triglyceride metabolism and normalizes elevated plasma glucose levels in mice. PLoS ONE 7, e37787 (2012).
Wu, Y. et al. Discovery of a highly potent, nonabsorbable apical sodium-dependent bile acid transporter inhibitor (GSK2330672) for treatment of type 2 diabetes. J. Med. Chem. 56, 5094–5114 (2013).
Balakrishnan, A. & Polli, J. E. Apical sodium dependent bile acid transporter (ASBT, SLC10A2): a potential prodrug target. Mol. Pharm. 3, 223–230 (2006).
McCracken, A. N. & Edinger, A. L. Nutrient transporters: the Achilles' heel of anabolism. Trends Endocrinol. Metab. 24, 200–208 (2013).
Ganapathy, V., Thangaraju, M. & Prasad, P. D. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 121, 29–40 (2009).
Chan, D. A. et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci. Transl. Med. 3, 94ra70 (2011).
Airley, R. E. & Mobasheri, A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy 53, 233–256 (2007).
Miranda-Goncalves, V. et al. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol. 15, 172–188 (2013).
Le Floch, R. et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl Acad. Sci. USA 108, 16663–16668 (2011).
Provost, J. J. & Wallert, M. A. Inside out: targeting NHE1 as an intracellular and extracellular regulator of cancer progression. Chem. Biol. Drug Des. 81, 85–101 (2013).
Loo, S. Y. et al. NHE-1: a promising target for novel anti-cancer therapeutics. Curr. Pharm. Des. 18, 1372–1382 (2012).
Imai, H. et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 30, 4819–4828 (2010).
Nawashiro, H. et al. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer 119, 484–492 (2006).
Hassanein, M. et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin. Cancer Res. 19, 560–570 (2013).
Timmerman, L. A. et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell 24, 450–465 (2013).
Gout, P. W., Buckley, A. R., Simms, C. R. & Bruchovsky, N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia 15, 1633–1640 (2001).
Kong, F.-L. & Yang, D. J. Amino acid transporter-targeted radiotracers for molecular imaging in oncology. Curr. Med. Chem. 19, 3271–3281 (2012).
Guan, Z., Xu, B., Wang, R., Sun, L. & Tian, J. Hyperaccumulation of 18F-FDG in order to differentiate solid pseudopapillary tumors from adenocarcinomas and from neuroendocrine pancreatic tumors and review of the literature. Hell. J. Nucl. Med. 16, 97–102 (2013).
Kaira, K., Sunaga, N., Ishizuka, T., Shimizu, K. & Yamamoto, N. The role of [18F]fluorodeoxyglucose positron emission tomography in thymic epithelial tumors. Cancer Imag. 11, 195–201 (2011).
Nogami, T. et al. Occupancy of serotonin and norepinephrine transporter by milnacipran in patients with major depressive disorder: a positron emission tomography study with [11C]DASB and (S,S)-[18F]FMeNER-D2 . Int. J. Neuropsychopharmacol. 16, 937–943 (2013).
Takano, A., Halldin, C. & Farde, L. SERT and NET occupancy by venlafaxine and milnacipran in nonhuman primates: a PET study. Psychopharmacology (Berl.) 226, 147–153 (2013).
Comley, R. A. et al. Monoamine transporter occupancy of a novel triple reuptake inhibitor in baboons and humans using positron emission tomography. J. Pharmacol. Exp. Ther. 346, 311–317 (2013).
Lin, S.-C. et al. In vivo detection of monoaminergic degeneration in early Parkinson disease by 18F-9-fluoropropyl-(+)-dihydrotetrabenzazine PET. J. Nucl. Med. 55, 73–79 (2014).
Giboureau, N., Som, I. M., Boucher-Arnold, A., Guilloteau, D. & Kassiou, M. PET radioligands for the vesicular acetylcholine transporter (VAChT). Curr. Top. Med. Chem. 10, 1569–1583 (2010).
Wulkersdorfer, B. et al. Using positron emission tomography to study transporter-mediated drug-drug interactions in tissues. Clin. Pharmacol. Ther. 96, 206–213 (2014).
Hume, W. E. et al. The synthesis and biodistribution of [11C]metformin as a PET probe to study hepatobiliary transport mediated by the multi-drug and toxin extrusion transporter 1 (MATE1) in vivo. Bioorg. Med. Chem. 21, 7584–7590 (2013).
Bamshad, M. J. et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat. Rev. Genet. 12, 745–755 (2011).
Seitz, S. et al. Pharmacological estrogen administration causes a FSH-independent osteo-anabolic effect requiring ER alpha in osteoblasts. PLoS ONE 7, e50301 (2012).
Smith, E. P. et al. Impact on bone of an estrogen receptor-α gene loss of function mutation. J. Clin. Endocrinol. Metab. 93, 3088–3096 (2008).
Brunham, L. R. & Hayden, M. R. Hunting human disease genes: lessons from the past, challenges for the future. Hum. Genet. 132, 603–617 (2013).
Stefanutti, C., Morozzi, C. & Di Giacomo, S. New clinical perspectives of hypolipidemic drug therapy in severe hypercholesterolemia. Curr. Med. Chem. 19, 4861–4868 (2012).
Kelley, R. I., Robinson, D., Puffenberger, E. G., Strauss, K. A. & Morton, D. H. Amish lethal microcephaly: a new metabolic disorder with severe congenital microcephaly and 2-ketoglutaric aciduria. Am. J. Med. Genet. 112, 318–326 (2002).
Rosenberg, M. J. et al. Mutant deoxynucleotide carrier is associated with congenital microcephaly. Nat. Genet. 32, 175–179 (2002).
Lindhurst, M. J. et al. Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc. Natl Acad. Sci. USA 103, 15927–15932 (2006).
Vallon, V. et al. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am. J. Physiol. Ren. Physiol. 302, F1293–F1299 (2012).
Chen, J. J. et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J. Neurosci. 21, 6348–6361 (2001).
Schmitt, A. et al. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J. Neurosci. Res. 71, 701–709 (2003).
Reidling, J. C., Lambrecht, N., Kassir, M. & Said, H. M. Impaired intestinal vitamin B1 (thiamin) uptake in thiamin transporter-2-deficient mice. Gastroenterology 138, 1802–1809 (2010).
Kono, S. et al. Mutations in a thiamine-transporter gene and Wernicke's-like encephalopathy. N. Engl. J. Med. 360, 1792–1794 (2009).
Debs, R. et al. Biotin-responsive basal ganglia disease in ethnic Europeans with novel SLC19A3 mutations. Arch. Neurol. 67, 126–130 (2010).
Paulusma, C. C. et al. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin–Johnson syndrome. Hepatology 25, 1539–1542 (1997).
Van de Steeg, E. et al. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J. Clin. Invest. 122, 519–528 (2012).
Gong, I. Y. & Kim, R. B. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab. Pharmacokinet. 28, 4–18 (2013).
Guan, J. et al. The xc− cystine/glutamate antiporter as a potential therapeutic target for small-cell lung cancer: use of sulfasalazine. Cancer Chemother. Pharmacol. 64, 463–472 (2009).
Reshkin, S. J., Cardone, R. A. & Harguindey, S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat. Anticancer Drug Discov. 8, 85–99 (2013).
Sundman-Eriksson, I., Blennow, K., Davidsson, P., Dandenell, A.-K. & Marcusson, J. Increased [3H]tiagabine binding to GAT-1 in the cingulate cortex in schizophrenia. Neuropsychobiology 45, 7–11 (2002).
Chue, P. Glycine reuptake inhibition as a new therapeutic approach in schizophrenia: focus on the glycine transporter 1 (GlyT1). Curr. Pharm. Des. 19, 1311–1320 (2013).
Daniels, R. W., Miller, B. R. & DiAntonio, A. Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol. Dis. 41, 415–420 (2011).
Hinoi, E., Takarada, T., Tsuchihashi, Y. & Yoneda, Y. Glutamate transporters as drug targets. Curr. Drug Targets CNS Neurol. Disord. 4, 211–220 (2005).
Lehenkari, P. P. et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 61, 1255–1262 (2002).
Lin, C. L. G. et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20, 589–602 (1998).
Yatomi, Y. et al. Chronic brain ischemia induces the expression of glial glutamate transporter EAAT2 in subcortical white matter. Neuroscience 244, 113–121 (2013).
Abrahamsen, B. et al. Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. J. Neurosci. 33, 1068–1087 (2013).
Huynh, T. H. et al. Structure–activity relationship study of selective excitatory amino acid transporter subtype 1 (EAAT1) inhibitor 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101) and absolute configurational assignment using infrared and vibrational circular dichroism spectroscopy in combination with ab initio Hartree–Fock calculations. J. Med. Chem. 55, 5403–5412 (2012).
Kanai, Y. et al. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 34, 108–120 (2013).
Takebayashi, R. et al. [18F] Fluorodeoxyglucose accumulation as a biological marker of hypoxic status but not glucose transport ability in gastric cancer. J. Exp. Clin. Cancer Res. 32, 34 (2013).
Mertens, K., Mees, G., Lambert, B., Van de Wiele, C. & Goethals, I. In vitro 2-deoxy-2-[18F]fluoro-D-glucose uptake: practical considerations. Cancer Biother. Radiopharm. 27, 183–188 (2012).
Liu, Y. et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol. Cancer Ther. 11, 1672–1682 (2012).
Zambrowicz, B. et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin. Pharmacol. Ther. 92, 158–169 (2012).
Gorboulev, V. et al. Na+–d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61, 187–196 (2012).
Feld, L. G. Renal glycosuria. Dayton Children's [online], (2003).
Weeks, A. J. et al. Evaluation of [18F]-tetrafluoroborate as a potential PET imaging agent for the human sodium/iodide symporter in a new colon carcinoma cell line, HCT116, expressing hNIS. Nucl. Med. Commun. 32, 98–105 (2011).
Jauregui-Osoro, M. et al. Synthesis and biological evaluation of [18F]tetrafluoroborate: a PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter. Eur. J. Nucl. Med. Mol. Imag. 37, 2108–2116 (2010).
Jeon, B. et al. Dopamine transporter imaging with [123I]-β-CIT demonstrates presynaptic nigrostriatal dopaminergic damage in Wilson's disease. J. Neurol. Neurosurg. Psychiatry 65, 60–64 (1997).
Jeon, B. S. et al. Dopamine transporter density measured by [123I]β-CIT single-photon emission computed tomography is normal in dopa-responsive dystonia. Ann. Neurol. 43, 792–800 (1998).
Morita, K. et al. Spinal antiallodynia action of glycine transporter inhibitors in neuropathic pain models in mice. J. Pharmacol. Exp. Ther. 326, 633–645 (2008).
Yoshikawa, S., Oguchi, T., Funahashi, Y., de Groat, W. C. & Yoshimura, N. Glycine transporter type 2 (GlyT2) inhibitor ameliorates bladder overactivity and nociceptive behavior in rats. Eur. Urol. 62, 704–712 (2012).
Kurosawa, Y. et al. Cyclocreatine treatment improves cognition in mice with creatine transporter deficiency. J. Clin. Invest. 122, 2837–2846 (2012).
Trotier-Faurion, A. et al. Synthesis and biological evaluation of new creatine fatty esters revealed dodecyl creatine ester as a promising drug candidate for the treatment of the creatine transporter deficiency. J. Med. Chem. 56, 5173–5181 (2013).
Mercimek-Mahmutoglu, S. et al. Treatment of intractable epilepsy in a female with SLC6A8 deficiency. Mol. Genet. Metab. 101, 409–412 (2010).
Sakamoto, S. et al. Identification of the transporters involved in the hepatobiliary transport and intestinal efflux of methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-naphthoate (S-8921) glucuronide, a pharmacologically active metabolite of S-8921. Drug Metab. Dispos. 36, 1553–1561 (2008).
Rais, R., Fletcher, S. & Polli, J. E. Synthesis and in vitro evaluation of gabapentin prodrugs that target the human apical sodium-dependent bile acid transporter (hASBT). J. Pharm. Sci. 100, 1184–1195 (2011).
Pinheiro, C. et al. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology 56, 860–867 (2010).
Kennedy, K. M. & Dewhirst, M. W. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Futur. Oncol. 6, 127–148 (2010).
Murray, C. M. et al. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat. Chem. Biol. 1, 371–376 (2005).
Polanski, R. et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 20, 926–937 (2014).
Patel, S. A., Nagy, J. O., Bolstad, E. D., Gerdes, J. M. & Thompson, C. M. Tetrapeptide inhibitors of the glutamate vesicular transporter (VGLUT). Bioorg. Med. Chem. Lett. 17, 5125–5128 (2007).
Carrigan, C. N. et al. Synthesis and in vitro pharmacology of substituted quinoline-2,4-dicarboxylic acids as inhibitors of vesicular glutamate transport. J. Med. Chem. 45, 2260–2276 (2002).
Zhang, Q. et al. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke's encephalopathy. Drug Metab. Dispos. 42, 1656–1662 (2014).
Iwai, N. et al. A high prevalence of renal hypouricemia caused by inactive SLC22A12 in Japanese. Kidney Int. 66, 935–944 (2004).
Iacobazzi, V. et al. Statins, fibrates and retinoic acid upregulate mitochondrial acylcarnitine carrier gene expression. Biochem. Biophys. Res. Commun. 388, 643–647 (2009).
Alasti, F., Van Camp, G. & Smith, R. J. Pendred syndrome/DFNB4. GeneReviews [online], (2014).
Soleimani, M. A novel target for diuretic therapy. Iran. J. Kidney Dis. 6, 419–425 (2012).
Bali, D. S., Chen, Y.-T. & Goldstein, J. L. Glycogen storage disease type I. GeneReviews [online], (2013).
Yiu, W. H. et al. Normoglycemia alone is insufficient to prevent long-term complications of hepatocellular adenoma in glycogen storage disease type Ib mice. J. Hepatol 51, 909–917 (2009) (2013).
Lee, Y. M. et al. Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology 56, 1719–1729 (2012).
Yang, Z. et al. Kinetics and specificity of feline leukemia virus subgroup C receptor (FLVCR) export function and its dependence on hemopexin. J. Biol. Chem. 285, 28874–28882 (2010).
Acknowledgements
The authors acknowledge the following funding sources: a US National Institutes of Health (NIH) Training Grant (T32 GM007175) to L.L; an NIH grant (GM61390) to S.W.Y.; an NIH Pharmacogenomics Research Network grant (GM61390) and a Burroughs Wellcome Fund Innovation in Regulatory Sciences grant (1012485, DK103729) to K.M.G.; and a Canadian Institutes of Health Research grant (MOP-89753, DSEN-PREVENT FRN-117588), the Ontario Institutes for Cancer Research, Cancer Care Ontario, and the Program of Experimental Medicine in the Department of Medicine at Western University, in Ontario, Canada, to R.B.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
K.M.G. is a co-founder of Apricity Therapeutics and has received grants from Pfizer, Sanofi–Aventis, AstraZeneca and GlaxoSmithKline, and has a patent pending. S.W.Y. is a co-founder of Apricity Therapeutics and has a patent pending. R.B.K. has a patent pending.
Related links
Supplementary information
Supplementary information S1 (table)
SLC transporter-associated Mendelian diseases. The transporters associated with Mendelian diseases and prevalence data on each disease are provided. (PDF 363 kb)
Supplementary information S2 (table)
SLC transporter genes and associated Mendelian diseases. (PDF 1152 kb)
Glossary
- Mendelian diseases
-
Disorders that are caused by mutations in a single gene and follow Mendelian inheritance patterns.
- Genome-wide association studies
-
(GWASs). Studies of multiple genetic variants across the genome in many individuals, looking for association with a given trait. In most GWASs, more than 500,000 genetic variants across the genome are examined for association with a certain trait of some individuals that does not appear in others.
- Z′ assay sensitivity factor
-
A measure of statistical effect size that takes into account the mean and standard deviation of both the positive and the negative controls.
- Pharmacophore modelling
-
Use of a geometric description of the chemical functions of a target protein to generate and use 3D structural information to search for novel active compounds. Models may be generated by either ligand-based or structure-based methods.
- Quantitative structure–activity relationship (QSAR) modelling
-
Use of a regression model to find relationships between the physical or chemical properties and the biological activity of a molecule, based on the assumption that these features are related.
- Docking
-
A computational method used to predict the orientation of molecules during interactions with a target protein.
- Homology models
-
Molecular models of a target protein created from its amino acid sequence and the 3D structure of a homologous protein.
Rights and permissions
About this article
Cite this article
Lin, L., Yee, S., Kim, R. et al. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov 14, 543–560 (2015). https://doi.org/10.1038/nrd4626
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4626
This article is cited by
-
Membrane transporters in drug development and as determinants of precision medicine
Nature Reviews Drug Discovery (2024)
-
MCT1-governed pyruvate metabolism is essential for antibody class-switch recombination through H3K27 acetylation
Nature Communications (2024)
-
Ion and lipid orchestration of secondary active transport
Nature (2024)
-
Impact of 1α,25-dihydroxyvitamin D3 on biodistribution and pharmacokinetics of L-carnitine and creatinine, organic cation/carnitine transporter 2 and organic cation transporter 2 biomarkers
Journal of Pharmaceutical Investigation (2024)
-
Low expression of SLC34A1 is associated with poor prognosis in clear cell renal cell carcinoma
BMC Urology (2023)