Key Points
-
Drug discovery approaches for cancer, as for other therapeutic areas, have typically been divided into two classes: target-based drug discovery (TDD) and phenotypic drug discovery (PDD). Cancer drug discovery poses substantial challenges for both targeted and 'classical' phenotypic drug discovery owing to the number, diversity and plasticity of molecular mechanisms and phenotypes underlying tumour initiation and growth.
-
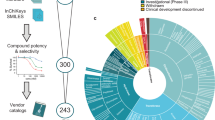
Discovery origins for all 48 small-molecule cancer drugs approved by the US Food and Drug Administration between 1999 and 2013, and for agents in Phase II and II clinical trials at the end of 2013, were analysed and classified.
-
Although a significant number of approved and investigational cancer drugs could be easily classified as targeted, the majority of which (21 out of 29) are kinase inhibitors, we concluded that very few drugs (four out of 48) were discovered entirely by 'classical' PDD. The remainder were discovered by, or developed from chemical lead matter discovered by a combination of phenotypic and target-based assays.
-
Drug discovery using cytoxicity assays and cancer cell lines, although yielding many of the current standard-of-care chemotherapies, is unlikely to result in further drugs with novel mechanisms of action.
-
Knowledge of the molecular pathways and targets required for specific disease-associated phenotypes, along with the ability to use more disease-relevant cell models, improves the probability of discovering drugs with novel mechanisms of action and clinical efficacy in molecularly defined patient populations.
-
We introduce the concept of 'mechanism-informed phenotypic drug discovery' (MIPDD) to include phenotypic assays for specific molecular pathways and targets. Determining the causal relationships between target inhibition and phenotypic effects may well open up new and unexpected avenues of cancer biology. Such an approach presents the best means of discovering drugs that have both an optimal molecular mechanism of action and a diagnostic hypothesis to enable patient selection leading to clinical responses.
Abstract
There has been a resurgence of interest in the use of phenotypic screens in drug discovery as an alternative to target-focused approaches. Given that oncology is currently the most active therapeutic area, and also one in which target-focused approaches have been particularly prominent in the past two decades, we investigated the contribution of phenotypic assays to oncology drug discovery by analysing the origins of all new small-molecule cancer drugs approved by the US Food and Drug Administration (FDA) over the past 15 years and those currently in clinical development. Although the majority of these drugs originated from target-based discovery, we identified a significant number whose discovery depended on phenotypic screening approaches. We postulate that the contribution of phenotypic screening to cancer drug discovery has been hampered by a reliance on 'classical' nonspecific drug effects such as cytotoxicity and mitotic arrest, exacerbated by a paucity of mechanistically defined cellular models for therapeutically translatable cancer phenotypes. However, technical and biological advances that enable such mechanistically informed phenotypic models have the potential to empower phenotypic drug discovery in oncology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Swinney, D. C. Phenotypic versus target-based drug discovery for first-in-class medicines. Clin. Pharmacol. Ther. 93, 299–301 (2013).
Sams-Dodd, F. Is poor research the cause of the declining productivity of the pharmaceutical industry? An industry in need of a paradigm shift. Drug Discov. Today 18, 211–217 (2013).
Sams-Dodd, F. Target-based drug discovery: is something wrong? Drug Discov. Today 10, 139–147 (2005).
Hopkins, A. L. Network pharmacology: the next paradigm in drug discovery. Nature Chem. Biol. 4, 682–690 (2008).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nature Rev. Drug Discov. 5, 993–996 (2006).
Butcher, E. C. Can cell systems biology rescue drug discovery? Nature Rev. Drug Discov. 4, 461–467 (2005).
Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nature Rev. Drug Discov. 9, 203–214 (2010).
Scannell, J. W., Blanckley, A., Boldon, H. & Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nature Rev. Drug Discov. 11, 191–200 (2012).
Bennani, Y. L. Drug discovery in the next decade: innovation needed ASAP. Drug Discov. Today 16, 779–792 (2011).
Hoelder, S., Clarke, P. A. & Workman, P. Discovery of small molecule cancer drugs: successes, challenges and opportunities. Mol. Oncol. 6, 155–176 (2012).
Lee, J. A., Uhlik, M. T., Moxham, C. M., Tomandl, D. & Sall, D. J. Modern phenotypic drug discovery is a viable, neoclassic pharma strategy. J. Med. Chem. 55, 4527–4538 (2012).
Swinney, D. C. & Anthony, J. How were new medicines discovered? Nature Rev. Drug Discov. 10, 507–519 (2011).
Arrowsmith, J. A decade of change. Nature Rev. Drug Discov. 11, 17–18 (2012).
Zhang, J., Yang, P. L. & Gray, N. S. Targeting cancer with small molecule kinase inhibitors. Nature Rev. Cancer 9, 28–39 (2009).
Garraway, L. A. & Lander, E. S. Lessons from the cancer genome. Cell 153, 17–37 (2013).
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nature Rev. Drug Discov. 1, 493–502 (2002).
Williams, R. Discontinued drugs in 2012: oncology drugs. Expert Opin. Investig. Drugs 22, 1627–1644 (2013).
Ellis, L. M. & Fidler, I. J. Finding the tumor copycat. Therapy fails, patients don't. Nature Med. 16, 974–975 (2010).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Carter, S. K. The search for therapeutic cell controls by the chemotherapy program of the National Cancer Institute. J. Invest. Dermatol. 59, 128–138 (1972).
DeVita, V. T. & Chu, E. A history of cancer chemotherapy. Cancer Res. 68, 8643–8653 (2008).
Kim, M.-J. et al. OPB-31121, a novel small molecular inhibitor, disrupts the JAK2/STAT3 pathway and exhibits an antitumor activity in gastric cancer cells. Cancer Lett. 335, 145–152 (2013).
Chan, D. A. & Giaccia, A. J. Harnessing synthetic lethal interactions in anticancer drug discovery. Nature Rev. Drug Discov. 10, 351–364 (2011).
Dolma, S., Lessnick, S. L., Hahn, W. C. & Stockwell, B. R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 (2003).
Shaw, A. T. et al. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl Acad. Sci. USA 108, 8773–8778 (2011).
Weïwer, M. et al. Development of small-molecule probes that selectively kill cells induced to express mutant RAS. Bioorg. Med. Chem. Lett. 22, 1822–1826 (2012).
Yoshida, T. et al. Identification and characterization of a novel chemotype MEK inhibitor able to alter the phosphorylation state of MEK1/2. Oncotarget 3, 1533–1545 (2012).
Teicher, B. A., Ara, G., Herbst, R., Palombella, V. J. & Adams, J. The proteasome inhibitor PS-341 in cancer therapy. Clin. Cancer Res. 5, 2638–2645 (1999).
O'Donnell, A. et al. Hormonal impact of the 17α-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br. J. Cancer 90, 2317–2325 (2004).
Boehm, M. F. et al. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J. Med. Chem. 38, 3146–3155 (1995).
Marks, P. A. & Breslow, R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nature Biotech. 25, 84–90 (2007).
Shortt, J., Hsu, A. K. & Johnstone, R. W. Thalidomide-analogue biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene 32, 4191–4202 (2013).
Lopez-Girona, A. et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26, 2326–2335 (2012).
Licht, J. D., Shortt, J. & Johnstone, R. From anecdote to targeted therapy: the curious case of thalidomide in multiple myeloma. Cancer Cell 25, 9–11 (2014).
Friend, C., Scher, W., Holland, J. G. & Sato, T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc. Natl Acad. Sci. USA 68, 378–382 (1971).
Ueda, H. et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J. Antibiot. 47, 301–310 (1994).
Nakajima, H., Kim, Y. B., Terano, H., Yoshida, M. & Horinouchi, S. FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp. Cell Res. 241, 126–133 (1998).
Hartford, C. M. & Ratain, M. J. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin. Pharmacol. Ther. 82, 381–388 (2007).
Kuhn, D. J. et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 110, 3281–3290 (2007).
Huang, M. T. Harringtonine, an inhibitor of initiation of protein biosynthesis. Mol. Pharmacol. 11, 511–519 (1975).
Jordan, M. A. et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 4, 1086–1095 (2005).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Wakeling, A. E. & Bowler, J. I.C. I. 182,780, a new antioestrogen with clinical potential. J. Steroid Biochem. Mol. Biol. 43, 173–177 (1992).
Lee, F. Y. F. et al. Preclinical discovery of ixabepilone, a highly active antineoplastic agent. Cancer Chemother. Pharmacol. 63, 157–166 (2008).
Galsky, M. D., Dritselis, A., Kirkpatrick, P. & Oh, W. K. Cabazitaxel. Nature Rev. Drug Discov. 9, 677–678 (2010).
Gandhi, V., Keating, M. J., Bate, G. & Kirkpatrick, P. Nelarabine. Nature Rev. Drug Discov. 5, 17–18 (2006).
Adjei, A. A. Pemetrexed: a multitargeted antifolate agent with promising activity in solid tumors. Ann. Oncol. 11, 1335–1341 (2000).
Giuliani, F. C. & Kaplan, N. O. New doxorubicin analogs active against doxorubicin-resistant colon tumor xenografts in the nude mouse. Cancer Res. 40, 4682–4687 (1980).
Gould, S. E. et al. Discovery and preclinical development of vismodegib. Expert Opin. Drug Discov. http://dx.doi.org/10.1517/17460441.2014.920816 (2014).
O'Dwyer, K. & Maslak, P. Azacitidine and the beginnings of therapeutic epigenetic modulation. Expert Opin. Pharmacother. 9, 1981–1986 (2008).
Iyer, R., Fetterly, G., Lugade, A. & Thanavala, Y. Sorafenib: a clinical and pharmacologic review. Expert Opin. Pharmacother. 11, 1943–1955 (2010).
Isaacs, J. T. The long and winding road for the development of tasquinimod as an oral second-generation quinoline-3-carboxamide antiangiogenic drug for the treatment of prostate cancer. Expert Opin. Investig. Drugs 19, 1235–1243 (2010).
Isaacs, J. T. et al. Tasquinimod is an allosteric modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 73, 1386–1399 (2013).
Yagoda, N. et al. RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447, 864–868 (2007).
Wong, H. et al. Antitumor activity of targeted and cytotoxic agents in murine subcutaneous tumor models correlates with clinical response. Clin. Cancer Res. 18, 3846–3855 (2012).
Wong, H. et al. Bridging the gap between preclinical and clinical studies using pharmacokinetic-pharmacodynamic modeling: an analysis of GDC-0973, a MEK inhibitor. Clin. Cancer Res. 18, 3090–3099 (2012).
Prinz, F., Schlange, T. & Asadullah, K. Believe it or not: how much can we rely on published data on potential drug targets? Nature Rev. Drug Discov. 10, 712–712 (2011).
Begley, C. G. & Ellis, L. M. Drug development: raise standards for preclinical cancer research. Nature 483, 531–533 (2012).
Swinney, D. C. The contribution of mechanistic understanding to phenotypic screening for first-in-class medicines. J. Biomol. Screen. 18, 1186–1192 (2013).
Mangana, J., Levesque, M. P., Karpova, M. B. & Dummer, R. Sorafenib in melanoma. Expert Opin. Investig. Drugs 21, 557–568 (2012).
Munshi, N. et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol. Cancer Ther. 9, 1544–1553 (2010).
Michieli, P. & Di Nicolantonio, F. Targeted therapies: Tivantinib — a cytotoxic drug in MET inhibitor's clothes? Nature Rev. Clin. Oncol. 10, 372–374 (2013).
Basilico, C. et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin. Cancer Res. 19, 2381–2392 (2013).
Katayama, R. et al. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res. 73, 3087–3096 (2013).
Reddy, M. V. R. et al. Discovery of a clinical stage multi-kinase inhibitor sodium (E)-2-{2-methoxy-5-[(2′,4“,6”-trimethoxystyrylsulfonyl)methyl]phenylamino}acetate (ON 01910. Na): synthesis, structure-activity relationship, and biological activity. J. Med. Chem. 54, 6254–6276 (2011).
Jimeno, A. et al. Phase I study of ON 01910. Na, a novel modulator of the Polo-like kinase 1 pathway, in adult patients with solid tumors. J. Clin. Oncol. 26, 5504–5510 (2008).
Roschewski, M., Farooqui, M., Aue, G., Wilhelm, F. & Wiestner, A. Phase I study of ON 01910. Na (Rigosertib), a multikinase PI3K inhibitor in relapsed/refractory B-cell malignancies. Leukemia 27, 1920–1923 (2013).
Prasad, A. et al. Styryl sulfonyl compounds inhibit translation of cyclin D1 in mantle cell lymphoma cells. Oncogene 28, 1518–1528 (2009).
Chapman, C. M. et al. ON 01910. Na is selectively cytotoxic for chronic lymphocytic leukemia cells through a dual mechanism of action involving PI3K/AKT inhibition and induction of oxidative stress. Clin. Cancer Res. 18, 1979–1991 (2012).
Nakahara, T. et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 67, 8014–8021 (2007).
Glaros, T. G. et al. The 'survivin suppressants' NSC 80467 and YM155 induce a DNA damage response. Cancer Chemother. Pharmacol. 70, 207–212 (2012).
Lewis, K. D. et al. A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest. New Drugs 29, 161–166 (2009).
Kelly, R. J. et al. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 24, 2601–2606 (2013).
Giaccone, G. et al. Multicenter Phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J. Clin. Oncol. 27, 4481–4486 (2009).
Coumar, M. S., Tsai, F.-Y., Kanwar, J. R., Sarvagalla, S. & Cheung, C. H. A. Treat cancers by targeting survivin: just a dream or future reality? Cancer Treat. Rev. 39, 802–811 (2013).
Ledford, H. Drug candidates derailed in case of mistaken identity. Nature 483, 519 (2012).
Hwang, S. G. et al. Anti-cancer activity of a novel small molecule compound that simultaneously activates p53 and inhibits NF-κB signaling. PLoS ONE 7, e44259 (2012).
Caie, P. D. et al. High-content phenotypic profiling of drug response signatures across distinct cancer cells. Mol. Cancer Ther. 9, 1913–1926 (2010).
Low, J. et al. Phenotypic fingerprinting of small molecule cell cycle kinase inhibitors for drug discovery. Curr. Chem. Genom. 3, 13–21 (2009).
Chan, G. K. Y., Kleinheinz, T. L., Peterson, D. & Moffat, J. G. A simple high-content cell cycle assay reveals frequent discrepancies between cell number and ATP and MTS proliferation assays. PLoS ONE 8, e63583 (2013).
Kimlin, L. C., Casagrande, G. & Virador, V. M. In vitro three-dimensional (3D) models in cancer research: an update. Mol. Carcinog. 52, 167–182 (2013).
Harrison, R. G. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 9, 787–846 (1910).
Drewitz, M. et al. Towards automated production and drug sensitivity testing using scaffold-free spherical tumor microtissues. Biotechnol. J. 6, 1488–1496 (2011).
Hsiao, A. Y. et al. Micro-ring structures stabilize microdroplets to enable long term spheroid culture in 384 hanging drop array plates. Biomed. Microdevices 14, 313–323 (2012).
LaBarbera, D. V., Reid, B. G. & Yoo, B. H. The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert Opin. Drug Discov. 7, 819–830 (2012).
Li, Q. et al. 3D models of epithelial-mesenchymal transition in breast cancer metastasis: high-throughput screening assay development, validation, and pilot screen. J. Biomol. Screen. 16, 141–154 (2011).
Korff, T. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 143, 1341–1352 (1998).
Friedrich, J., Seidel, C., Ebner, R. & Kunz-Schughart, L. A spheroid-based drug screen: considerations and practical approach. Nature Protoc. 4, 309–324 (2009).
Misund, K. et al. A method for measurement of drug sensitivity of myeloma cells co-cultured with bone marrow stromal cells. J. Biomol. Screen 18, 637–646 (2013).
Haglund, C. et al. In vitro evaluation of clinical activity and toxicity of anticancer drugs using tumor cells from patients and cells representing normal tissues. Cancer Chemother. Pharmacol. 69, 697–707 (2012).
Carmody, L. C. et al. Phenotypic high-throughput screening elucidates target pathway in breast cancer stem cell-like cells. J. Biomol. Screen 17, 1204–1210 (2012).
Valent, P. et al. Cancer stem cell definitions and terminology: the devil is in the details. Nature Rev. Cancer 12, 767–775 (2012).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10, 717–728 (2012).
Romaguera-Ros, M. et al. Cancer-initiating enriched cell lines from human glioblastoma: preparing for drug discovery assays. Stem Cell Rev. 8, 288–298 (2012).
Lee, T. K. W., Cheung, V. C. H. & Ng, I. O. L. Liver tumor-initiating cells as a therapeutic target for hepatocellular carcinoma. Cancer Lett. 338, 101–109 (2013).
Izrailit, J. & Reedijk, M. Developmental pathways in breast cancer and breast tumor-initiating cells: therapeutic implications. Cancer Lett. 317, 115–126 (2012).
Morrison, B. J., Morris, J. C. & Steel, J. C. Lung cancer-initiating cells: a novel target for cancer therapy. Target Oncol. 8, 159–172 (2013).
Sharma, S. V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Yan, H. et al. Drug-tolerant cancer cells show reduced tumor-initiating capacity: depletion of CD44 cells and evidence for epigenetic mechanisms. PLoS ONE 6, e24397 (2011).
Lee, G.-Y. et al. Stochastic acquisition of a stem cell-like state and drug tolerance in leukemia cells stressed by radiation. Int. J. Hematol. 93, 27–35 (2011).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Cheng, Z. et al. Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin. Cancer Res. 19, 1748–1759 (2013).
Arrowsmith, C. H., Bountra, C., Fish, P. V., Lee, K. & Schapira, M. Epigenetic protein families: a new frontier for drug discovery. Nature Rev. Drug Discov. 11, 384–400 (2012).
Qian, J., Lu, L., Wu, J. & Ma, H. Development of multiple cell-based assays for the detection of histone H3 Lys27 trimethylation (H3K27me3). Assay Drug Dev. Technol. 11, 449–456 (2013).
Mulji, A. et al. Configuration of a high-content imaging platform for hit identification and pharmacological assessment of JMJD3 demethylase enzyme inhibitors. J. Biomol. Screen. 17, 108–120 (2012).
Snuderl, M. et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 20, 810–817 (2011).
Sottoriva, A. et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl Acad. Sci. USA 110, 4009–4014 (2013).
Jiang, P. et al. Novel anti-glioblastoma agents and therapeutic combinations identified from a collection of FDA approved drugs. J. Transl. Med. 12, 13 (2014).
Joshi, A. D. et al. Evaluation of tyrosine kinase inhibitor combinations for glioblastoma therapy. PLoS ONE 7, e44372 (2012).
Engström, P. G. et al. Digital transcriptome profiling of normal and glioblastoma-derived neural stem cells identifies genes associated with patient survival. Genome Med. 4, 76 (2012).
Vitucci, M. et al. Cooperativity between MAPK and PI3K signaling activation is required for glioblastoma pathogenesis. Neuro-Oncol. 15, 1317–1329 (2013).
Kaur, G. et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J. Natl Cancer Inst. 84, 1736–1740 (1992).
Bax, D. A. et al. Molecular and phenotypic characterisation of paediatric glioma cell lines as models for preclinical drug development. PLoS ONE 4, e5209 (2009).
Breuleux, M. et al. BAL27862: a unique microtubule destabilizer active against chemorefractory breast cancers. Cancer Res. 69 (Suppl. 24), 2093 (2010).
Joo, K. M. et al. Patient-specific orthotopic glioblastoma xenograft models recapitulate the histopathology and biology of human glioblastomas in situ. Cell Rep. 3, 260–273 (2013).
Danovi, D., Folarin, A. A., Baranowski, B. & Pollard, S. M. High content screening of defined chemical libraries using normal and glioma-derived neural stem cell lines. Methods Enzymol. 506, 311–329 (2012).
Mendelsohn, J. Personalizing oncology: perspectives and prospects. J. Clin. Oncol. 31, 1904–1911 (2013).
Sha, S.-K. et al. Cell cycle phenotype-based optimization of G2-abrogating peptides yields CBP501 with a unique mechanism of action at the G2 checkpoint. Mol. Cancer Ther. 6, 147–153 (2007).
Hangauer, D. G. Compositions for treating cell proliferation disorders. US Patent 7300931 (2007).
Dvorakova, K. et al. Induction of oxidative stress and apoptosis in myeloma cells by the aziridine-containing agent imexon. Biochem. Pharmacol. 60, 749–758 (2000).
Vidal, A. et al. Lurbinectedin (PM01183), a new DNA minor groove binder, inhibits growth of orthotopic primary graft of cisplatin-resistant epithelial ovarian cancer. Clin. Cancer Res. 18, 5399–5411 (2012).
Hayakawa, F. et al. A novel STAT inhibitor, OPB-31121, has a significant antitumor effect on leukemia with STAT-addictive oncokinases. Blood Cancer J. 3, e166–e169 (2013).
Guirouilh-Barbat, J., Antony, S. & Pommier, Y. Zalypsis (PM00104) is a potent inducer of γ-H2AX foci and reveals the importance of the C ring of trabectedin for transcription-coupled repair inhibition. Mol. Cancer Ther. 8, 2007–2014 (2009).
Wiman, K. G. Pharmacological reactivation of mutant p53: from protein structure to the cancer patient. Oncogene 29, 4245–4252 (2010).
Kirshner, J. R. et al. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther. 7, 2319–2327 (2008).
Sahasrabudhe, S. R. et al. Selective in vitro and in vivo anti-tumor activity of PRLX 93936 in biological models of melanoma and ovarian cancer. J. Clin. Oncol. 26, 14586 (2008).
Funahashi, Y. et al. Sulfonamide derivative, E7820, is a unique angiogenesis inhibitor suppressing an expression of integrin α2 subunit on endothelium. Cancer Res. 62, 6116–6123 (2002).
Chau, C. H. & Figg, W. D. New tricks from an old drug: a role for quinacrine in anti-cancer therapy? Cell Cycle 8, 4024–4025 (2009).
Gumireddy, K. et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell 7, 275–286 (2005).
Hawtin, R. E. et al. Voreloxin is an anticancer quinolone derivative that intercalates DNA and poisons topoisomerase II. PLoS ONE 5, e10186 (2010).
Tozer, G. M. et al. Combretastatin A-4 phosphate as a tumor vascular-targeting agent: early effects in tumors and normal tissues. Cancer Res. 59, 1626–1634 (1999).
Urdiales, J., Morata, P., De Castro, I. N. & Sánchez-Jiménez, F. Antiproliferative effect of dehydrodidemnin B (DDB), a depsipeptide isolated from Mediterranean tunicates. Cancer Lett. 102, 31–37 (1996).
Takahashi-Yanaga, F. & Kahn, M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin. Cancer. Res. 16, 3153–3162 (2010).
Robarge, K. D. et al. GDC-0449 — a potent inhibitor of the hedgehog pathway. Bioorg. Med. Chem. Lett. 19, 5576–5581 (2009).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chem. Biol. 5, 100–107 (2009).
Yamaguchi, T. et al. Identification of JTP-70902, a p15(INK4b)-inductive compound, as a novel MEK1/2 inhibitor. Cancer Sci. 98, 1809–1816 (2007).
Li, H. et al. Versatile pathway-centric approach based on high-throughput sequencing to anticancer drug discovery. Proc. Natl Acad. Sci. USA 109, 4609–4614 (2012).
Stoops, S. L. et al. Identification and optimization of small molecules that restore E-cadherin expression and reduce invasion in colorectal carcinoma cells. ACS Chem. Biol. 6, 452–465 (2011).
Lavelin, I. et al. Discovery of novel proteasome inhibitors using a high-content cell-based screening system. PLoS ONE 4, e8503 (2009).
Zhang, L. et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl Acad. Sci. USA 104, 19023–19028 (2007).
Roller, D. G. et al. Synthetic lethal screening with small-molecule inhibitors provides a pathway to rational combination therapies for melanoma. Mol. Cancer Ther. 11, 2505–2515 (2012).
McLaughlin, J. et al. Preclinical characterization of Aurora kinase inhibitor R763/AS703569 identified through an image-based phenotypic screen. J. Cancer Res. Clin. Oncol. 136, 99–113 (2010).
Mayer, T. U. et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971–974 (1999).
Guzi, T. J. et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol. Cancer Ther. 10, 591–602 (2011).
Quintavalle, M., Elia, L., Price, J. H., Heynen-Genel, S. & Courtneidge, S. A. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Sci. Signal. 4, ra49 (2011).
Lee, J. et al. A small molecule inhibitor of α4 integrin-dependent cell migration. Bioorg. Med. Chem. 17, 977–980 (2009).
Yarrow, J. C., Totsukawa, G., Charras, G. T. & Mitchison, T. J. Screening for cell migration inhibitors via automated microscopy reveals a Rho-kinase inhibitor. Chem. Biol. 12, 385–395 (2005).
Stevens, M. F. & Newlands, E. S. From triazines and triazenes to temozolomide. Eur. J. Cancer 29A, 1045–1047 (1993).
Gottardis, M. M. et al. Chemoprevention of mammary carcinoma by LGD1069 (Targretin): an RXR-selective ligand. Cancer Res. 56, 5566–5570 (1996).
Buchdunger, E. et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 56, 100–104 (1996).
Arteaga, C. L. & Johnson, D. H. Tyrosine kinase inhibitors-ZD1839 (Iressa). Curr. Opin. Oncol. 13, 491–498 (2001).
Carson, D. A. et al. Oral antilymphocyte activity and induction of apoptosis by 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine. Proc. Natl Acad. Sci. USA 89, 2970–2974 (1992).
Taylor, E. C. et al. A dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5- yl)ethyl]benzoyl]-l-glutamic acid, is an inhibitor of thymidylate synthase. J. Med. Chem. 35, 4450–4454 (1992).
Moyer, J. D. et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 57, 4838–4848 (1997).
Giudici, D. et al. 6-methylenandrosta-1,4-diene-3, 17-dione (FCE 24304): a new irreversible aromatase inhibitor. J. Steroid Biochem. 30, 391–394 (1988).
Kotla, V. et al. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2, 36 (2009).
Gandhi, V. et al. Compound GW506U78 in refractory hematologic malignancies: relationship between cellular pharmacokinetics and clinical response. J. Clin. Oncol. 16, 3607–3615 (1998).
Lombardo, L. J. et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 47, 6658–6661 (2004).
Sun, L. et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl- 1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J. Med. Chem. 46, 1116–1119 (2003).
Weisberg, E. et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 7, 129–141 (2005).
Wilhelm, S. & Chien, D.-S. BAY 43-9006: preclinical data. Curr. Pharm. Des. 8, 2255–2257 (2002).
Leoni, L. M. et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin. Cancer Res. 14, 309–317 (2008).
Podar, K. et al. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proc. Natl Acad. Sci. USA 103, 19478–19483 (2006).
Xia, W. et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21, 6255–6263 (2002).
Potter, G. A., Barrie, S. E., Jarman, M. & Rowlands, M. G. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J. Med. Chem. 38, 2463–2471 (1995).
Cui, J. J. et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 54, 6342–6363 (2011).
Quintás-Cardama, A. et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115, 3109–3117 (2010).
Hennequin, L. F. et al. Novel 4-anilinoquinazolines with C-7 basic side chains: design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J. Med. Chem. 45, 1300–1312 (2002).
Bollag, G. et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nature Rev. Drug Discov. 11, 873–886 (2012).
Inai, T. et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am. J. Pathol. 165, 35–52 (2004).
Puttini, M. et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 66, 11314–11322 (2006).
Zhang, Y., Guessous, F., Kofman, A., Schiff, D. & Abounader, R. XL-184, a MET, VEGFR-2 and RET kinase inhibitor for the treatment of thyroid cancer, glioblastoma multiforme and NSCLC. IDrugs 13, 112–121 (2010).
Demo, S. D. et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 67, 6383–6391 (2007).
Huang, W.-S. et al. Discovery of 3-[2-(imidazo[1,2-b]pyridazin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide (AP24534), a potent, orally active pan-inhibitor of breakpoint cluster region-abelson (BCR-ABL) kinase including the T315I gatekeeper mutant. J. Med. Chem. 53, 4701–4719 (2010).
Li, D. et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27, 4702–4711 (2008).
Medina, T., Amaria, M. N. & Jimeno, A. Dabrafenib in the treatment of advanced melanoma. Drugs Today 49, 377–385 (2013).
Honigberg, L. A. et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl Acad. Sci. USA 107, 13075–13080 (2010).
Acknowledgements
The authors thank R. Blake, J. Lee, S. Malek, M. Prunotti and D. Swinney for stimulating discussions, and D. Lowe, B. Roth and the anonymous internal and external reviewers for their extremely constructive comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.G.M. and J.R. own stock in Hoffman-La Roche.
Related links
Rights and permissions
About this article
Cite this article
Moffat, J., Rudolph, J. & Bailey, D. Phenotypic screening in cancer drug discovery — past, present and future. Nat Rev Drug Discov 13, 588–602 (2014). https://doi.org/10.1038/nrd4366
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4366
This article is cited by
-
VIBRANT: spectral profiling for single-cell drug responses
Nature Methods (2024)
-
A statistical framework for high-content phenotypic profiling using cellular feature distributions
Communications Biology (2022)
-
Reactions of cisplatin and oxaliplatin with penicillin G: implications for drug inactivation and biological activity
JBIC Journal of Biological Inorganic Chemistry (2022)
-
Identification of anticancer drug target genes using an outside competitive dynamics model on cancer signaling networks
Scientific Reports (2021)
-
Drug combination screening as a translational approach toward an improved drug therapy for chordoma
Cellular Oncology (2021)