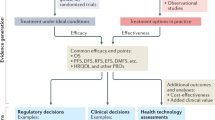
Abstract
The flow of new medicines to patients depends on the development of new biomarkers and their correct interpretation, yet there are no widely accepted and practically applicable criteria that facilitate adequate biomarker qualification. As a result, case-by-case qualifications are based on subjective assessments that do not lead to optimal decisions for patients, which have contributed to the 'stagnation' in drug productivity identified by the FDA. An alternative is to qualify biomarkers in terms of cost effectiveness using a set of principles that enable the evaluation of biomarkers even with incomplete knowledge. This approach could minimize harm to patients, improve access to medicines and reduce healthcare costs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
US FDA. The Critical Path to New Medicinal Products [online], <http://www.fda.gov/oc/initiatives/criticalpath/>
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 8, 431–40 (1989)
Katz, R. Biomarkers and surrogate markers: an FDA perspective. J. American Soc. Exp. NeuroTher. 1, 189–195 (2004).
Boissel, J.-P., Collet, J.-P., Moleur, P. & Haugh, M. Surrogate endpoints, a basis for a rational approach. Eur. J. Clin. Pharm. 43, 235–244 (1992).
European Medicines Agency. Note for Guidance on Statistical Principles for Clinical Trials [online], <http://www.emea.eu.int/pdfs/human/ich/036396en.pdf> (1998).
Lesko, L. & Atkinson, A. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu. Rev. Pharmacol. Toxicol. 41, 347–366 (2001).
Graham, J. D. & Rhomberg, L. in Challenges in Risk Assessment and Risk Management (eds Kunreuther, H. & Slovic, P.) 15–24 (Sage, Thousand Oaks, 1996)
OECD. Emerging Systemic Risks. Final report to the OECD Future Project p67 (OECD, Paris 2003)
Klinke, A. & Renn, O. A new approach to Risk evaluation and management: risk-based, precaution-based and discourse based management. Risk Anal. 22, 1071–1094 (2002).
Lofstedt, R. Risk Management in Post Trust Societies (Palgrave Macmillan, London, 2005).
Slovic, P. The Perception of Risk (Earthscan, London, 2000).
Branca, M. A new perspective on pharmacogenomics in drug development. SPECTRUM: Therapy Markets and Emerging Technologies (2004).
Fischoff, B., Slovic, P. & Lichtenstein, L How safe is safe enough? A psychometric study of attitudes towards technological risks and benefits. Policy Sci. 9, 127–152 (1978).
UK Health & Safety Executive. Reducing Risks, Protecting People: HSE's Decision-Making Process [online], <http://www.hse.gov.uk/risk/theory/r2p2.pdf#search=%22HSE%3A%20Reducing%20risks%3A%20protecting%20people.%20(Health%20and%20Safety%20Executive%20London%202001)%22> (2001).
UK HM Treasury. Managing Risks To The Public: Appraisal Guidance [online], <http://www.hm-treasury.gov.uk/media/8AB/54/Managing_risks_to_the_public.pdf> (2005).
Devlin, M. & Parkin, D. Who evaluates NICE? BMJ 327, 1061–1062 (2003).
FDA. Challenge and Opportunity on the Critical Path to New Medical Products (2004).
Editorial. Fewer new drugs from the pharmaceutical industry. BMJ 326, 408–409 (2003).
Lofstedt, R. E. Risk communication and Cox-2: can pharma do better? J. Risk Commun. (in the press).
Leaf, C. How our national obsession with drug safety is killing people — and what we can do about it. Fortune (9 February 2006).
Navarro, V. J. & Senior, J. R. Drug related hepatotoxicity. N. Engl. J. Med. 354, 731–739 (2006).
Espeland, M. et al. Carotid intima-medial thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr. Control. Trials Cardiovasc.Med. 6, 1–6 (2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.A.W. and D.E.S. are employees of and stockholders in Pfizer, Inc. J.A.W. is an employee of and stockholder in Merck & Co. C.J.W. is an employee of and stockholder in Millennium Pharmaceuticals.
Related links
Rights and permissions
About this article
Cite this article
Williams, S., Slavin, D., Wagner, J. et al. A cost-effectiveness approach to the qualification and acceptance of biomarkers. Nat Rev Drug Discov 5, 897–902 (2006). https://doi.org/10.1038/nrd2174
Issue Date:
DOI: https://doi.org/10.1038/nrd2174
This article is cited by
-
Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives
Nature Reviews Drug Discovery (2010)
-
A multistep validation process of biomarkers for preclinical drug development
The Pharmacogenomics Journal (2010)
-
The Value, Qualification, and Regulatory Use of Surrogate End Points in Drug Development
Clinical Pharmacology & Therapeutics (2009)
-
A Prototypical Process for Creating Evidentiary Standards for Biomarkers and Diagnostics
Clinical Pharmacology & Therapeutics (2008)
-
Biomarker Development, Commercialization, and Regulation: Individualization of Medicine Lost in Translation
Clinical Pharmacology & Therapeutics (2007)