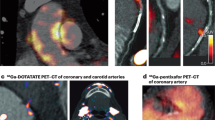
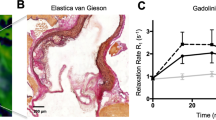
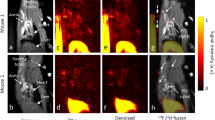
Key Points
-
Atherosclerosis is a systemic disease that is characterized by the build-up of lipid-rich plaques within the walls of large arteries, and underlies the clinical conditions of myocardial infarction, chronic stable angina, stroke and peripheral vascular disease.
-
However, until recently, there has been no effective way to detect the presence of atherosclerosis in patients until it has reached a relatively advanced stage, and so a large window of opportunity for primary prevention through lifestyle modification and drug therapy targeted at individuals with sub-clinical disease is missed.
-
The appreciation that a considerable atherosclerotic burden, including plaques vulnerable to rupture or susceptible to erosion, can be accommodated in the vessel wall without impingement on the lumen has led to a new imperative for direct plaque imaging. In addition, an increasing appreciation of the molecular and cellular events involved in atherothrombosis opens up new vistas for targeted imaging.
-
This article reviews approaches for imaging atherosclerosis, including magnetic resonance imaging and intravascular ultrasound. For both clinical and research applications, imaging techniques allow non-invasive appraisal of disease processes and provide the potential for serial monitoring in the same individual.
-
The careful selection of validated imaging endpoints will allow clinical trials to be conducted more quickly and often with many fewer patients than are required for conventional 'clinical outcome' studies.
Abstract
Recent years have seen a dramatic expansion in our knowledge of the events of atherogenesis and in the availability of drugs that can retard the progression — and even induce the regression — of this disease process. Our understanding has been advanced considerably by developments in genetics and molecular biology and by the use of genetically modified mouse models that have provided key mechanistic insights. Increasingly sophisticated imaging techniques will capitalize on these advances by bringing forward diagnosis, enhancing disease characterization and providing more precise evaluation of the effects of treatment. In this review, techniques for imaging atherosclerosis and thrombosis will be discussed. Particular attention will be given to magnetic resonance imaging techniques that enable lesion characterization and allow the targeted imaging of cells, molecules and biological processes. Emphasis is given to the potential contribution of magnetic resonance imaging methods to therapeutic monitoring, drug delivery and drug discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
McGill, H. C. Jr. & McMahan, C. A. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Am. J. Cardiol. 82, 30T–36T (1998).
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Davies, M. J., Richardson, P. D., Woolf, N., Katz, D. R. & Mann, J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br. Heart J. 69, 377–381 (1993).
Glagov, S., Weisenberg, E., Zarins, C. K., Stankunavicius, R. & Kolettis, G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316, 1371–1375 (1987).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Glass, C. K. & Witztum, J. L. Atherosclerosis. the road ahead. Cell 104, 503–516 (2001).
Fayad, Z. A. & Fuster, V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ. Res. 89, 305–316 (2001).
Nissen, S. E. & Yock, P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 103, 604–616. (2001).
Barnett, H. J. et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 339, 1415–1425 (1998).
Excecutive Committee for the Asymtomatic Carotid Atherosclerosis Study. Endarterectomy for asymtomatic carotid stenosis. J. Am. Med. Assoc. 273, 1421–14228 (1995).
Weinberger, J., Ramos, L., Ambrose, J. A. & Fuster, V. Morphologic and dynamic changes of atherosclerotic plaque at the carotid artery bifurcation: sequential imaging by real time B-mode ultrasonography. J. Am. Coll. Cardiol. 12, 1515–1521 (1988).
Pignoli, P., Tremoli, E., Poli, A., Oreste, P. & Paoletti, R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 74, 1399–1406 (1986).
Fazio, G. P., Redberg, R. F., Winslow, T. & Schiller, N. B. Transesophageal echocardiographically detected atherosclerotic aortic plaque is a marker for coronary artery disease. J. Am. Coll. Cardiol. 21, 144–150 (1993).
O'Leary, D. H. et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N. Engl. J. Med. 340, 14–22. (1999).
Khoury, Z. et al. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid, and femoral arteries evaluated by ultrasound. Am. J. Cardiol. 80, 1429–1433 (1997).
Burke, G. L. et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 26, 386–391 (1995).
Nagai, Y. et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation 98, 1504–1509 (1998).
Crouse, J. R. 3rd, Craven, T. E., Hagaman, A. P. & Bond, M. G. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation 92, 1141–1147 (1995).
Salonen, R. et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 92, 1758–1764 (1995).
de Groot, E. et al. B-mode ultrasound assessment of pravastatin treatment effect on carotid and femoral artery walls and its correlations with coronary arteriographic findings: a report of the Regression Growth Evaluation Statin Study (REGRESS). J. Am. Coll. Cardiol. 31, 1561–1567 (1998).
Weinberger, J., Azhar, S., Danisi, F., Hayes, R. & Goldman, M. A new noninvasive technique for imaging atherosclerotic plaque in the aortic arch of stroke patients by transcutaneous real-time B-mode ultrasonography: an initial report. Stroke 29, 673–676 (1998).
The French study of aortic plaques in stroke group. Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. N. Engl. J. Med. 334, 1216–1221 (1996).
Cohen, A. et al. Aortic plaque morphology and vascular events: a follow-up study in patients with ischemic stroke. FAPS Investigators. French Study of Aortic Plaques in Stroke. Circulation 96, 3838–3841 (1997).
Parthenakis, F. et al. Absence of atherosclerotic lesions in the thoracic aorta indicates absence of significant coronary artery disease. Am. J. Cardiol. 77, 1118–1121 (1996).
Lanza, G. M. et al. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation 94, 3334–3340 (1996).
Lindner, J. R. et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 102, 2745–2750 (2000).
Demos, S. M. et al. In vivo targeting of acoustically reflective liposomes for intravascular and transvascular ultrasonic enhancement. J. Am. Coll. Cardiol. 33, 867–875 (1999).
Tiukinhoy, S. D., Huang, S., Khan, A. A., MacDonald, R. C. & McPherson, D. D. Novel acoustic drug-encapsulated liposomes for site-specific delivery. J. Am. Coll. Cardiol. 37, 256A (2001).
Tiukinhoy, S. D. et al. Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Invest Radiol 35, 732–738 (2000).
Skinner, M. P. et al. Serial magnetic resonance imaging of experimental atherosclerosis detects lesion fine structure, progression and complications in vivo. Nat Med 1, 69–73 (1995).
Helft, G. et al. Atherosclerotic aortic component quantification by noninvasive magnetic resonance imaging: an in vivo study in rabbits. J. Am. Coll. Cardiol. 37, 1149–1154 (2001).
Fayad, Z. A. et al. In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation 101, 2503–2509 (2000).
Toussaint, J. F., LaMuraglia, G. M., Southern, J. F., Fuster, V. & Kantor, H. L. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 94, 932–938 (1996). This landmark paper demonstrated the ability of MRI to characterize atherosclerotic lesions in humans in vivo.
Hatsukami, T. S., Ross, R., Polissar, N. L. & Yuan, C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 102, 959–964 (2000).
Yuan, C. et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 104, 2051–2056 (2001).
Yuan, C. et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 105, 181–185 (2002).
Moody, A. R., Allder, S., Lennox, G., Gladman, J. & Fentem, P. Direct magnetic resonance imaging of carotid artery thrombus in acute stroke. Lancet 353, 122–123 (1999).
Botnar, R. M., Stuber, M., Danias, P. G., Kissinger, K. V. & Manning, W. J. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation 99, 3139–3148 (1999).
Botnar, R. M. et al. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation 102, 2582–2587 (2000).
Fayad, Z. A. et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 102, 506–510 (2000). This paper showed that is possible to overcome the many limitations to apply MRI to visualize coronary arterial wall.
Botnar, R. M. et al. 3D coronary vessel wall imaging utilizing a local inversion technique with spiral image acquisition. Magn. Reson. Med. 46, 848–854 (2001).
Mani, V. et al. Rapid extended coverage (REX) simultaneous multislice black blood vessel wall imaging. Radiology 232, 281–288 (2004).
Itskovich, V. V. et al. Parallel and nonparallel simultaneous multislice black-blood double inversion recovery techniques for vessel wall imaging. J. Magn. Reson. Imaging 19, 459–467 (2004).
Worthley, S. G. et al. A novel nonobstructive intravascular MRI coil: in vivo imaging of experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 346–350 (2003).
Yuan, C. et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J. Magn. Reson. Imaging 15, 62–67 (2002).
Kerwin, W. et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 107, 851–856 (2003).
Kramer, C. M. et al. Magnetic resonance imaging identifies the fibrous cap in atherosclerotic abdominal aortic aneurysm. Circulation 109, 1016–1021 (2004).
Barkhausen, J., Ebert, W., Heyer, C., Debatin, J. F. & Weinmann, H. -J. Detection of atherosclerotic plaque with gadofluorine-enhanced magnetic resonance imaging. Circulation 108, 605–609 (2003).
Topol, E. J. & Nissen, S. E. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 92, 2333–2342 (1995).
Corti, R. et al. Lipid Lowering by simvastatin induces regression of human atherosclerotic lesions: two years' follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation 106, 2884–2887 (2002). Second in a series of two papers using MRI to demonstrate human atherosclerosis regression with statin treatment.
Corti, R. et al. The selective peroxisomal proliferator-activated receptor-γ agonist has an additive effect on plaque regression in combination with simvastatin in experimental atherosclerosis: in vivo study by high-resolution magnetic resonance imaging. J. Am. Coll. Cardiol. 43, 464–473 (2004).
Nissen, S. E. et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290, 2292–2300 (2003).
Nissen, S. E. et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291, 1071–1080 (2004).
Zhao, X. Q. et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler. Thromb. Vasc. Biol. 21, 1623–1629 (2001). Although a case-control study, in a relatively small number of patients, this report demonstrated that in addition to plaque size, it is also possible to quantify individual plaque components and to identify changes in plaque composition with intensive lipid-lowering therapy.
Plump, A. S., Scott, C. J. & Breslow, J. L. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl Acad. Sci. USA 91, 9607–9611 (1994).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).
Kusunoki, J. et al. Acyl-CoA:cholesterol acyltransferase inhibition reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 103, 2604–2609 (2001).
Shah, P. K. et al. High-dose recombinant apolipoprotein A-i(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein E-deficient mice: potential implications for acute plaque stabilization. Circulation 103, 3047–3050 (2001).
Sparrow, C. P. et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler. Thromb. Vasc. Biol. 21, 115–121 (2001).
Reis, E. D. et al. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J. Vasc. Surg. 34, 541–547 (2001).
Rong, J. X. et al. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation 104, 2447–2452 (2001).
Fayad, Z. A. et al. Noninvasive In vivo high-resolution magnetic resonance imaging of atherosclerotic lesions in genetically engineered mice. Circulation 98, 1541–1547 (1998). Mice have become the pre-eminent animal model for the study of atherosclerosis. This was the first of a number of studies that identified atherosclerosis in mice in vivo using high-field-strength MRI.
Choudhury, R. P. et al. Atherosclerotic lesions in genetically modified mice quantified in vivo by non-invasive high-resolution magnetic resonance microscopy. Atherosclerosis 162, 315–321 (2002).
Aguinaldo, J. G. S. et al. Localization of a novel contrast agent gadofluorine on atheroslcerotic plaque of apolipoprotein E knockout mouse using in vivo magnetic resonance microscopy. Proc. Intl Soc. Magn. Reson. Med. A1699 (2004).
Wiesmann, F. et al. High-resolution MRI with cardiac and respiratory gating allows for accurate in vivo atherosclerotic plaque visualization in the murine aortic arch. Magn. Reson. Med. 50, 69–74 (2003).
Hockings, P. D. et al. Repeated three-dimensional magnetic resonance imaging of atherosclerosis development in innominate arteries of low-density lipoprotein receptor-knockout mice. Circulation 106, 1716–1721 (2002).
Choudhury, R. P. et al. Serial, noninvasive, in vivo magnetic resonance microscopy detects the development of atherosclerosis in apolipoprotein E-deficient mice and its progression by arterial wall remodeling. J. Magn. Reson. Imaging 17, 184–189 (2003).
Litovsky, S. et al. Superparamagnetic iron oxide-based method for quantifying recruitment of monocytes to mouse atherosclerotic lesions in vivo: enhancement by tissue necrosis factor-α, interleukin-1β, and interferon-γ. Circulation 107, 1545–1549 (2003).
McAteer, M. A. et al. Quantification and 3D reconstruction of atherosclerotic plaque components in apolipoprotein E knockout mice using ex vivo high-resolution MRI. Arterioscler. Thromb. Vasc. Biol. (in the press).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Lusis, A. J. Atherosclerosis. Nature 407, 233–241 (2000).
Gimbrone, M. A. Jr., Topper, J. N., Nagel, T., Anderson, K. R. & Garcia-Cardena, G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. NY Acad. Sci. 902, 230–239; discussion 239–240 (2000).
Celermajer, D. S. et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340, 1111–1115 (1992).
Sorensen, M. B. et al. Long-term use of contraceptive depot medroxyprogesterone acetate in young women impairs arterial endothelial function assessed by cardiovascular magnetic resonance. Circulation 106, 1646–1651 (2002).
Suwaidi, J. A. et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101, 948–954 (2000).
Halcox, J. P. et al. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J. Am. Coll. Cardiol. 40, 1232–1240 (2002).
Heitzer, T., Schlinzig, T., Krohn, K., Meinertz, T. & Munzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104, 2673–2678 (2001).
Bonetti, P. O., Lerman, L. O. & Lerman, A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 23, 168–175 (2003).
Haffner, S. M., Lehto, S., Ronnemaa, T., Pyorala, K. & Laakso, M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 339, 229–234 (1998).
Steinberg, H. O. et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 97, 2601–2610 (1996).
O'Driscoll, G. et al. Improvement in endothelial function by angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 33, 1506–1511 (1999).
Cheetham, C. et al. Losartan, an angiotensin type 1 receptor antagonist, improves endothelial function in non-insulin-dependent diabetes. J. Am. Coll. Cardiol. 36, 1461–1466 (2000).
Tsunekawa, T. et al. Cerivastatin, a hydroxymethylglutaryl coenzyme A reductase inhibitor, improves endothelial function in elderly diabetic patients within 3 days. Circulation 104, 376–379 (2001).
Avogaro, A. et al. Gemfibrozil improves insulin sensitivity and flow-mediated vasodilatation in type 2 diabetic patients. Eur. J. Clin. Invest. 31, 603–609 (2001).
Wilson, P. W., Abbott, R. D. & Castelli, W. P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 8, 737–741 (1988).
Lupattelli, G. et al. Direct association between high-density lipoprotein cholesterol and endothelial function in hyperlipemia. Am. J. Cardiol. 90, 648–650 (2002).
Bisoendial, R. J. et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation 107, 2944–2948 (2003).
Weissleder, R. & Mahmood, U. Molecular imaging. Radiology 219, 316–333 (2001).
Wickline, S. A. & Lanza, G. M. Molecular imaging, targeted therapeutics, and nanoscience. J. Cell. Biochem. Suppl. 39, 90–97 (2002).
Wickline, S. A. & Lanza, G. M. Nanotechnology for molecular imaging and targeted therapy. Circulation 107, 1092–1095 (2003).
Rudin, M. & Weissleder, R. Molecular imaging in drug discovery and development. Nature Rev. Drug Discov. 2, 123–131 (2003).
Jaffer, F. A. & Weissleder, R. Seeing within: molecular imaging of the cardiovascular system. Circ. Res. 94, 433–445 (2004).
Runge, V. M. & Nelson, K. M. in Magnetic Resonance Imaging Vol. 1 (eds Stark, D. D. & Bradley, W. G.) (Mosby, St Louis, 1999).
Merbach, A. E. & Toth, E. (eds). The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging (John Wiley and Sons, Chichester, 2001).
Sipkins, D. A. et al. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nature Med. 4, 623–626 (1998). An early report of targeted molecular imaging using MRI.
Yu, X. et al. High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn. Reson. Med. 44, 867–872 (2000).
Winter, P. M. et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted nanoparticles. Circulation 108, 2270–2274 (2003).
Flacke, S. et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation 104, 1280–1285 (2001). An elegant demonstration of the use of gadolinium–DTPA-loaded nanoparticles to image fibrin and therefore identify thrombus in vivo.
Johansson, L. O., Bjornerud, A., Ahlstrom, H. K., Ladd, D. L. & Fujii, D. K. A targeted contrast agent for magnetic resonance imaging of thrombus: implications of spatial resolution. J. Magn. Reson. Imaging 13, 615–618 (2001).
Fayad, Z. A. et al. Detection of arterial thrombi in vivo by MRI using a fibrin-targeted contrast agent. Circulation 106, AII-435 (2002).
Cybulsky, M. I. & Gimbrone, M. A. Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251, 788–791 (1991).
Nakashima, Y., Raines, E. W., Plump, A. S., Breslow, J. L. & Ross, R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 18, 842–851 (1998).
Davies, M. J. et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J. Pathol. 171, 223–229 (1993).
Iiyama, K. et al. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ. Res. 85, 199–207 (1999).
Sipkins, D. A. et al. ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J. Neuroimmunol. 104, 1–9 (2000).
Sibson, N. R. et al. MRI detection of early endothelial activation in brain inflammation. Magn. Reson. Med. 51, 248–252 (2004).
Stary, H. C. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 15, 1512–1531 (1995).
Galis, Z. S., Sukhova, G. K., Kranzhofer, R., Clark, S. & Libby, P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc. Natl Acad. Sci. USA 92, 402–406 (1995).
Aguinaldo, J. G. S. et al. Atheroslcerotic plaque imaging using a novel contrast agent gadofluorine M. Mol. Imaging 2, 282 (2003).
Sirol, M. et al. Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation 109, 2890–2896 (2004).
Moreno, P. R. et al. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation 90, 775–778 (1994).
Schmitz, S. A. et al. Superparamagnetic iron oxide-enhanced MRI of atherosclerotic plaques in Watanabe hereditable hyperlipidemic rabbits. Invest. Radiol. 35, 460–471 (2000).
Ruehm, S. G., Corot, C., Vogt, P., Kolb, S. & Debatin, J. F. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation 103, 415–422 (2001).
Kooi, M. E. et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107, 2453–2458 (2003).
Louie, A. Y. et al. In vivo visualization of gene expression using magnetic resonance imaging. Nature Biotechnol. 18, 321–325 (2000). A report of an extremely elegant construct that is activated in vivo by enzymatic cleavage that exposes gadolinium to the aqueous environment in which it is active as a contrast agent. 'Smart' contrast agents of this sort present exciting possibilities for functional imaging of enzyme activity and biological processes.
Arap, W. et al. Steps toward mapping the human vasculature by phage display. Nature Med. 8, 121–127 (2002).
Zurita, A. J., Arap, W. & Pasqualini, R. Mapping tumor vascular diversity by screening phage display libraries. J. Control. Release 91, 183–186 (2003).
Kaul, S. & Lindner, J. R. Visualizing coronary atherosclerosis in vivo: thinking big, imaging small. J. Am. Coll. Cardiol. 43, 461–463 (2004).
Hamilton, A. J. et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J. Am. Coll. Cardiol. 43, 453–460 (2004).
Lindner, J. R. et al. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 104, 2107–2112 (2001).
Leong-Poi, H., Christiansen, J., Klibanov, A. L., Kaul, S. & Lindner, J. R. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to αv-integrins. Circulation 107, 455–460 (2003).
Falati, S., Gross, P., Merrill-Skoloff, G., Furie, B. C. & Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nature Med. 8, 1175–1181 (2002).
Chen, J. et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation 105, 2766–2771 (2002).
Jaffer, F. A., Tung, C. -H., Gerszten, R. E. & Weissleder, R. In vivo imaging of thrombin activity in experimental thrombi with thrombin-sensitive near-infrared molecular probe. 22, 1929–1935 (2002).
Bremer, C., Tung, C. H. & Weissleder, R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nature Med. 7, 743–748 (2001).
Vallabhajosula, S. & Fuster, V. Atherosclerosis: imaging techniques and the evolving role of nuclear medicine. J. Nucl. Med. 38, 1788–1796 (1997).
Iuliano, L. et al. Preparation and biodistribution of 99m technetium labelled oxidized LDL in man. Atherosclerosis 126, 131–141 (1996).
Iuliano, L., Mauriello, A., Sbarigia, E., Spagnoli, L. G. & Violi, F. Radiolabeled native low-density lipoprotein injected into patients with carotid stenosis accumulates in macrophages of atherosclerotic plaque: effect of vitamin E supplementation. Circulation 101, 1249–1254 (2000).
Hardoff, R. et al. External imaging of atherosclerosis in rabbits using an 123I-labeled synthetic peptide fragment. J. Clin. Pharmacol. 33, 1039–1047 (1993).
Tsimikas, S. et al. Radiolabeled MDA2, an oxidation-specific, monoclonal antibody, identifies native atherosclerotic lesions in vivo. J. Nucl. Cardiol. 6, 41–53 (1999).
Rudd, J. H. F. et al. Imaging atherosclerotic plaque inflammation with [18f]-fluorodeoxyglucose positron emission tomography. Circulation 105, 2708–2711 (2002). Preliminary, but interesting, report on the use of positron emission tomography for imaging inflamed atherosclerotic plaque.
Sharma, V., Luker, G. D. & Piwnica-Worms, D. Molecular imaging of gene expression and protein function in vivo with PET and SPECT. J. Magn. Reson. Imaging 16, 336–351 (2002).
Tatsumi, M., Cohade, C., Nakamoto, Y. & Wahl, R. L. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology 229, 831–837 (2003).
Helft, G. et al. Non-invasive in vivo imaging of atherosclerotic lesions using fluorine-18 deoxyglucose (18-FDG) PET correlates with macrophage content in a rabbit model. Circulation 100, I–311 (1999).
Lanza, G. M. et al. Targeted antiproliferative drug delivery to vascular smooth muscle cells with a magnetic resonance imaging nanoparticle contrast agent: implications for rational therapy of restenosis. Circulation 106, 2842–2847 (2002).
Anderson, S. A. et al. Magnetic resonance contrast enhancement of neovasculature with αvβ3-targeted nanoparticles. Magn. Reson. Med. 44, 433–439 (2000).
Acknowledgements
This work was supported in part by NIH/NHLBI R01 HL071021 (ZAF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
FURTHER INFORMATION
Glossary
- PLAQUES
-
Lesions within the wall of a large artery that contain high levels of lipids, lipoproteins, macrophage- derived foam cells, lymphocytes and smooth muscle cells. Advanced lesions can be covered by a fibrous cap that can rupture or erode, leading to thrombosis formation and vessel occlusion.
- MYOCARDIAL INFARCTION
-
Commonly known as a heart attack, this is the death of part of the heart muscle due to sudden loss of blood supply. Typically, the loss of this supply is caused by a complete blockage of a coronary artery by a blood clot.
- ECHOGENICITY
-
The degree to which sound waves are reflected by a tissue (and, by implication, the corresponding brightness on the visual representation).
- STENOSIS
-
The narrowing of a blood vessel, often due to the build-up of plaque.
- INTIMA
-
The innermost layer of arteries.
- MEDIA
-
The middle layer of arteries that lies between the intima and the adventitia and which normally comprises well-ordered layers of smooth muscle cells.
- CONTRAST AGENTS
-
Compounds that enhance the differences between or within tissues in imaging studies and which are often used to highlight abnormalities.
- RELAXATION TIMES
-
In magnetic resonance, after radiofrequency excitation, the return of nuclei to an equilibrium state within the static magnetic field is associated with loss of transverse magnetization (with time constant T2) and return of longitudinal magnetization (with time constant T1).
- EXTERNAL ELASTIC LAMINA
-
Concentric layers of elastic membranes that separate the media from the adventitia in arteries.
- STATIN
-
Drugs in the statin class inhibit a key enzyme in cholesterol biosynthesis — 3-hydroxy-3-methyl-glutaryl-CoA reductase — and reduce plasma levels of low-density lipoprotein cholesterol.
- VOXEL
-
A three-dimensional analogue of a pixel.
- Z-STACKS
-
Three-dimensional constructs made by sequential aggregation of images from spatially adjacent [x,y] planes.
- APOLIPOPROTEIN E KNOCKOUT MOUSE
-
This genetically modified mouse lacks apolipoprotein E, a ligand for low-density-lipoprotein receptors. As a consequence, these mice become hypercholesterolaemic and develop spontaneous atherosclerosis, which has some features in common with the human disease. This mouse had become the pre-eminent animal model for the study of atherosclerosis.
Rights and permissions
About this article
Cite this article
Choudhury, R., Fuster, V. & Fayad, Z. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov 3, 913–925 (2004). https://doi.org/10.1038/nrd1548
Issue Date:
DOI: https://doi.org/10.1038/nrd1548
This article is cited by
-
αvβ3 Integrin Receptor Targeting and Near-Infrared Imaging of Solid Tumors Using Surface-Modified Nanoliposomes
Journal of Pharmaceutical Innovation (2018)
-
MRI detection of endothelial cell inflammation using targeted superparamagnetic particles of iron oxide (SPIO)
Clinical and Translational Medicine (2017)
-
Dual targeting improves capture of ultrasound microbubbles towards activated platelets but yields no additional benefit for imaging of arterial thrombosis
Scientific Reports (2017)
-
Discovery of novel peptides targeting pro-atherogenic endothelium in disturbed flow regions -Targeted siRNA delivery to pro-atherogenic endothelium in vivo
Scientific Reports (2016)
-
Molecular Cardiovascular Magnetic Resonance: Current Status and Future Prospects
Current Cardiology Reports (2016)