Abstract
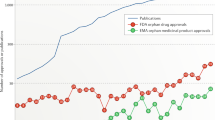
The process of clinical development and regulatory review of new therapeutics in the United States was significantly changed by a number of legislative acts passed in the 1980s and 1990s. These acts were designed to encourage the development of innovative products, especially for rare, serious or life-threatening diseases, and to ensure that patients had timely access to these treatments. To assess the effects of the various modifications to the process, the Tufts Center for the Study of Drug Development analysed clinical development and approval data for 554 therapeutics (504 small molecules, 40 recombinant proteins and 10 monoclonal antibodies) approved in the United States from 1980–2001. Trends in the number of approved products and the clinical development and approval times indicated that the effects of these changes were generally beneficial as of the mid- to late-1990s, but that the gains have not been sustained in the early 2000s. Current efforts by the FDA, and the pharmaceutical and biopharmaceutical industry, to reverse the recent tendency toward fewer new approvals and longer approval times are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lasagna, L. The development and regulation of new medicines. Science 200, 871–873 (1978).
Severson, J. A. Hearing Testimony Oversight hearing on 'Gene Patents and Other Genomic Inventions,' House Committee on the Judiciary, Subcommittee on Courts and Intellectual Property, July 13, 2000. J. Assoc. Univ. Technol. Managers 12, 1–8 (2000).
Milne, C. -P. Orphan products-pain relief for clinical development headaches. Nature Biotechnol. 20, 780–784 (2002).
Shulman, S. R., DiMasi, J. A. & Kaitin, K. I. Patent term restoration: the impact of the Waxman–Hatch Act on new drugs and biologics approved 1984–1995. J. Biolaw Bus. 2, 63–68 (1999).
Milne, C. -P. & Bergman, E. Fast track product designation under the Food and Drug Administration Modernization Act: the industry experience. Drug Inf. J. 35, 71–83 (2001).
Reichert, J. M., Chee, J. & Kotzampaltiris, C. S. The effects of the Prescription Drug User Fee Act and the Food and Drug Administration Modernization Act on the development and approval of therapeutic medicines. Drug Inf. J. 35, 85–94 (2001).
Reichert, J. M. & Paquette, C. Therapeutic recombinant proteins: trends in US approvals 1982–2002. Curr. Opin. Mol. Ther. 5, 139–147 (2003).
Reichert, J. M. Therapeutic monoclonal antibodies: trends in development and approval in the US. Curr. Opin. Mol. Ther. 4, 110–118 (2002).
DiMasi, J. A. New drug development in the United States 1963–1999. Clin. Pharmacol. Ther. 69, 286–296 (2001).
Kaitin, K. I. & Cairns, C. The new drug approvals of 1999, 2000, and 2001: drug development trends a decade after passage of the user fee act. Drug Inf. J. (in the press).
Kaitin, K. I. & Healy, E. M. The new drug approvals of 1996, 1997, and 1998: drug development trends in the user fee era. Drug Inf. J. 34, 1–14 (2000).
Kaitin, K. I. & Manocchia, M. A. The new drug approvals of 1993, 1994, and 1995: trends in drug development. Am. J. Ther. 4, 46–54 (1997).
Kaitin, K. I., Manocchia, M., Seibring, M. & Lasagna, L. The new drug approvals of 1990, 1991, and 1992: trends in drug development. J. Clin. Pharmacol. 34, 120–127 (1994).
Kaitin, K. I., DiCerbo, P. A. & Lasagna, L. The new drug approvals of 1987, 1988, and 1989: trends in drug development. J. Clin. Pharmacol. 31, 116–122 (1991).
Kaitin, K. I., Richard, B. W. & Lasagna, L. Trends in drug development: the 1985–86 new drug approvals. J. Clin. Pharmacol. 27, 542–548 (1987).
US FDA. Improving Innovation in Medical Technology: Beyond 2002 [online], (cited 04 August 2003), <http://www.fda.gov/bbs/topics/news/2003/beyond2002/report.html> (2002).
Acknowledgements
The author gratefully acknowledges the work of Elaine Bergman, Catherine Cairns and Cherie Paquette in maintaining the marketed new chemical entity and biopharmaceutical databases, and thanks Drs Kenneth Kaitin and Joseph DiMasi for helpful comments and suggestions.
Author information
Authors and Affiliations
Related links
Related links
FURTHER INFORMATION
Center for Biologics Evaluation and Research
Glossary
- CLINICAL PHASE I
-
Studies performed in a small number of patients or normal volunteers to evaluate pharmacokinetic properties (absorption, distribution, metabolism and excretion in humans) and establish a safe dosage range.
- CLINICAL PHASE II
-
Trials performed in a small number of patients to evaluate the safety and effectiveness of a product in comparison with a standard or control treatment.
- CLINICAL PHASE III
-
Large trials performed in patients to confirm safety, efficacy and optimal dosage range of a product in comparison with a standard or control for treatment of the targeted indication.
- INVESTIGATIONAL NEW DRUG APPLICATION
-
(IND). Document filed by a drug sponsor to notify the Food and Drug Administration of its intent to conduct clinical studies in human subjects.
- NEW DRUG APPLICATION, BIOLOGICS LICENSE APPLICATION, PREMARKET APPROVAL APPLICATION
-
(NDA, BLA, PMA). Marketing applications that contain the results of clinical Phase I, II and III studies and are submitted to the FDA for review. If the applications are approved, the product can be marketed in the United States.
Rights and permissions
About this article
Cite this article
Reichert, J. Trends in development and approval times for new therapeutics in the United States. Nat Rev Drug Discov 2, 695–702 (2003). https://doi.org/10.1038/nrd1178
Issue Date:
DOI: https://doi.org/10.1038/nrd1178