Key Points
-
It is estimated that only 1 out of 5,000 screened compounds is approved as a new medicine. Success or failure in drug development often depends on selecting one or two molecules for development from many choices offered by the engines of high-throughput discovery.
-
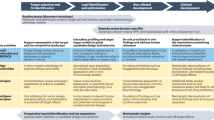
Drug development is a process that proceeds through several key go/no-go 'decision gates', from the identification of a potential therapeutic candidate through to marketing a drug product.
-
Success rests not only in the intrinsic qualities of the molecule but also in how the drug's development is planned and executed, and in the effective management of key resources: effort, time and cost.
-
Non-clinical studies form the basis for confidence in the safe and efficient progression of a new chemical entity into clinical testing. New in vitro methodologies that predict human response, coupled with time-tested protocols for drug testing in live animals and the emergence of sensitive analytical instrumentation and molecular genetics, play important roles in bringing safe and efficacious new drug candidates to market.
-
This review discusses how to strategically identify which non-clinical studies should be performed to provide the required guidance and comfort to stakeholders involved in clinical drug testing.
Abstract
Drug development is a risky business. Success or failure often depends on selecting one or two molecules for development from many choices offered by the engines of high-throughput discovery. A lead candidate needs to possess adequate bioactivity, appropriate physical–chemical properties to enable formulation development, the ability to cross crucial membranes, reasonable metabolic stability and appropriate safety and efficacy in humans. Predicting how a drug will behave in humans before clinical testing requires a battery of sophisticated in vitro tests that complement traditional in vivo animal safety assessments. This review discusses how to strategically identify which non-clinical studies should be performed to provide the required guidance and comfort to stakeholders involved in clinical drug testing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Guidance for Industry: S7A Safety Pharmacology Studies for Human Pharmaceuticals. US Food and Drug Administration, July 2001 <http://www.fda.gov/cder/4461fnl.pdf> (2001).
ICH Final Draft Guidance for Non-Clinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals: Proposal for revision. 8 November 2000.
Pharmaceutical Research and Manufacturers of America (PhRMA), Phrma FAX of the Week, May 2, 2003, R&D Process: Long, Expensive, Risky Road.
Lin, J. H. & Lu, A. Y. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol. Rev. 49, 403–449 (1997).
Gunaratna, C. Drug metabolism & pharmacokinetics in drug discovery: a primer for bioanalytical chemists, part I. Curr. Sep. 19, 17–23 (2000). This articles provides a good introduction to the fundamentals of drug metabolism
Kapur, R. Smarter lead optimization enabled. Drug Discov. Dev. 61. (1999).
Lead optimization strategies. Genet. Eng. News 19, 16 (1999).
Benet, L. Z. & Hoener, B. A. Changes in plasma protein binding have little clinical relevance. Clin. Pharmacol. Ther. 71, 115–121 (2002).
Artursson, P. Epithelial transport of drugs in cell culture I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79, 476–482 (1990).
Irvine, J. D. et al. MDCK (Madin–Darby canine kidney) cells: A tool for membrane permeability screening. J. Pharm. Sci. 88, 28–33 (1997).
Inui, K. -I., Yamamoto, M. & Saito, H. Transepithelial transport of oral cephalosporins by monolayers of intestinal epithelial cell line Caco-2: specific transport systems in apical and basolateral membranes. J. Pharmacol. Exp. Ther. 261, 195–201 (1992).
Lu, S., Guttendorf, R. J. & Stewart, B. H. The mechanism of cefdinir transport in CaCO-2 cells. Pharm. Res. 11, S–258 (1994).
Artursson, P. & Borchardt, R. T. Intestinal drug absorption and metabolism in cell cultures: Caco-2 and beyond. Pharm. Res. 14, 1655–1658 (1997).
Balkovetz, D. F. Hepatocyte growth factor and Madin–Darby canine kidney cells: in vitro models of epithelial cell movement and morphogenesis. Microsc. Res. Tech. 43, 456–463 (1998).
Kansey, M. et al. Physiochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 41, 1007–1010 (2001).
Sugano, K., Hamada, H., Machida, M. & Ushio, H. High throughput prediction of oral absorption: improvement of the composition of the lipid solution used in parallel artificial membrane permeation assay. J. Biomol. Screen. 16, 189–196 (2001).
Baillie, T. A. et al. Drug metabolites in safety testing. Toxicol. Appl. Pharmacol. 182, 188–196 (2002). A good paper the emphasizes the importance of understanding the safety of the products of drug metabolism.
Hawksworth, G. M. Advantages and disadvantages of using human cells for pharmacological and toxicological studies. Hum. Exp. Tox. 13, 568–573 (1994).
Roberts, S. A. Drug metabolism and pharmacokinetics in drug discovery. Curr. Opin. Drug Discov. Devel. 6, 66–80 (2003).
de Groene, E. M. et al. Development of human cytochrome P450-expressing cell lines: application in mutagenicity testing of ochratoxin A. Cancer Res. 56, 299–303 (1996).
de Groene, E. M. in Cytochrome P450 Protocols Methods in Molecular Biology (eds Shephard, E. A. & Phillips, E. A.) 219–226 (1998)
Li, A. P. et al. Present status of the application of cryopreserved hepatocytes in the evaluation of xenobiotics: consensus of an international expert panel. Chem. Biol. Interact. 121, 117–123 (1999).
Li, A. P. et al. Cryopreserved human hepatocytes: characterization of drug-metabolizing enzyme activities and applications in higher throughput screening assays for hepatotoxicity, metabolic stability, and drug–drug interaction potential. Chem. Biol. Interact. 121, 17–35 (1999).
White, R. E. High-throughput screening in drug metabolism and pharmacokinetic support of drug discovery. Annu. Rev. Pharmacol. Toxicol. 40, 133–157 (2000).
Roberts, S. A. High-throughput screening approaches for investigating drug metabolism and pharmacokinetics. Xenobiotica 31, 557–589 (2001).
Eichelbaum, M. Polymorphic drug oxidation in humans. Fed Proc. 43, 2298–2392 (1984).
Dayer, P. et al. Characterization of a common genetic defect of cytochrome P-450 function (debrisoquine-sparteine type polymorphism) — increased Michaelis is Constant (Km) and loss of stereoselectivity of bufuralol 1'-hydroxylation in poor metabolizers. Biochem. Biophys. Res. Commun. 125, 374–380 (1984).
Pettipher, R. The role of genomics in the development of new and improved therapies: An appreciation of the importance of genetic diversity will have a profound impact on how drugs are discovered, developed and prescribed. Innov. Pharm. Technol. 62–67 (2000).
Kuehl, P. et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature Genet. 27, 383–391 (2001).
Guengerich, F. P. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 39, 1–17 (1999).
Guidance for Industry: Drug Metabolism/Drug Interaction Studies in the Drug Development Process: Studies In Vitro (1997) Food and Drug Administration: <http://www.fda.gov/cder/guidance/clin3.pdf> (1997)
Clancy, C. E. & Rudy, Y., Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature 400, 566–569 (1999).
Bian, J., Cui, J. & McDonald, T. V. HERG K(+) channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ. Res. 89, 1168–1176 (2001).
Zhou, Z., Gong, Q., Epstein, M. L. January, C. T. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J. Biol. Chem. 273, 21061–21066 (1998).
Ulrich, R. & Friend, S. H. Toxicogenomics and drug discovery: will new technologies help us produce better drugs?. Nature Rev. Drug Discov. 1, 84–88 (2002). This is an opinion article that explores the changes that toxicogenomics might have on the cost and speed of drug development.
Schmidt, C. W. Toxicogenomics: an emerging discipline. Environ. Health Perspect. 110, A750–A755 (2002). This article explains the how toxicogenomics could impact drug development and environmental health assessments of low-level exposure limits.
Thayer, A. M. Genomics moves on. Chem. Eng. News 80, 25–36 (2002).
Therapeutic Products Directorate Guidance: Drug–Drug Interaction Studies: In Vivo and In Vitro. Health Canada Website: http://www.hc-sc.gc.ca (2000).
Guidance for Industry: In Vivo Drug Metabolism/Drug Interaction Studies — Study Design, Data Analysis, and Recommendations for Dosing and Labelling. US Food and Drug Administration <http://www.fda.gov/cder/guidance/2635.pdf> (1999).
Huang, S. -M. et al. Assessment of the quality and quantity of drug–drug interaction studies in recent NDA submissions: Study design and data analysis issues. J. Clin. Pharmacol. 39, 1006–1014 (1999).
Guidance for Industry: Good Laboratory Practice for Nonclinical Laboratory Studies (21 CFR Part 58) US Food and Drug Administration. http://www.fda.goc/cder
Eddershaw, P. J., Beresford, A. P. & Bayliss, M K. ADME/PK as part of a rational approach to drug discovery. Drug Discov. Today 5, 409–414 (2000).
Guidance for Industry: Good Laboratory Practice for Nonclinical Laboratory Studies (21 CFR Part 58) Food and Drug Administration Homepage. <http://www.fda.goc/cder>
Wilding, I. Injecting innovation into the drug development process. Scripps Magazine 15–16, October (2002).
Bayliss, M. K. & Frick, L. W. High-throughput pharmacokinetics: Cassette doisng. Curr. Opin. Drug Discov. Devel. 2, 20–25 (1999).
Cayen, M. N. Considerations in the design of toxicokinetic programs. Tox. Path. 23, 148–157 (1995).
Guideline for Industry: Detection of Toxicity to Reproduction for Medicinal Products. Food and Drug Administration.
Gonzalez, F. J. Overview of experimental approaches for study of drug metabolism and drug-drug interactions. Adv. Pharmacol. 43, 255–277 (1997).
Guideline for Industry: Pharmacokinetics: Guidance for Repeated Dose Tissue Distribution Studies. US Food and Drug Administration <http://www.fda.gov/cder/guidance/ichs3b.pdf> (1995)
Guidance for Industry: S4ADuration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Testing). US Food and Drug Adminsitration. <www.fda.gov/cder/guidance/62599.pdf> (1999).
Guidance for Industry: Immunotoxicology Evaluation of Investigational New Drugs, Issued October 2002, Food and Drug Administration. <http://www.fda.gov/cder/guidance/4945fnl.doc> (2002)
ICH Draft Consensus Guideline: Safety Pharmacology Studies For Assessing The Potential For Delayed Ventricular Repolarization (Qt Interval Prolongation) By Human Pharmaceuticals, February 2002.
Beckwith-Hall, B. M. et al. Nuclear magnetic spectroscopic and principal components analysis investigations into biochemical effects of three model hepatotoxins. Chem. Res. Tox. 11, 260–272 (1998).
Scarfe, G. B. et al. 19F NMR and directly coupled HPLC-NMR-MS investigations into the metabolism of 2-bromo-4-trifluoromethylaniline in the rat: a urinary excretion balance study without the use of radiolabelling. Xenobiotica 28, 373–388 (1998).
Cherry, S. R. et al. MicroPET: a high resolution PET scanner for imaging small animals. IEEE Trans. Nucl. Sci. 44, 1161–1166 (1997).
Paans, A. M. J. & Vaalburg, W. Positron emission tomography in drug development and drug evaluation. Curr. Pharm. Des. 6, 1583–1591 (2000). This paper explores the emerging use of PET imaging to support traditional ADME/PK and toxicology investigations supporting drug development.
Benet, L. Z. & Hoener, B. A. Changes in plasma protein binding have little clinical relevance. Clin. Pharmacol. Ther. 71, 115–121 (2002).
Spahn Langguth, H. et al. P-glycoprotein transporters and the gastrointestinal tract: evaluation of the potential in vivo relevance of in vitro data employing talinolol as a model compound. Int. J. Clin. Pharmacol. Ther. 36, 16–24 (1998).
Ramaswamy, M. et al. Influence of phytostanol phosphoryl ascorbate, FM-VP4, on pancreatic lipase activity and cholesterol accumulation within Caco-2 cells. J. Pharm. Pharm. Sci. 5, 29–38 (2002).
Tucker, G. T., Houston, J. B. & Huang, S. -M., Optimizing drug development: strategies to assess drug metabolism/transporter interaction potential — toward a consensus. Clin. Pharmacol. Ther. 70, 103–114 (2001).
Moore, L. B. et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl Acad. Sci. USA 97, 7500–7502 (2000).
Li, A. P. Primary hepatocyte cultures as an in vitro experimental system for the evaluation of pharmacokinetic drug–drug interactions. Adv. Pharmacol. 43, 103–130 (1997).
Lehmann, J. M. et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 102, 1016–1023 (1998).
Goodwin, B., Hodgson, E. & Liddle, C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol. Pharmacol. 56, 1329–1339 (1999).
Ogg, M. S. et al. A reporter gene assay to assess the molecular mechanisms of xenobiotic-dependent induction of the human CYP3A4 gene in vitro. Xenobiotica 29, 269–279 (1999).
Luo, G. et al. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and Cyp3A4 expression in human hepatoyctes. Drug Metab. Dispos. 30, 795–804 (2002).
Baillie, T. A. et al. Drug metabolites in safety testing. Toxicol. Appl. Pharmacol. 182, 188–196 (2002).
Guideline for Industry: The Need for Long–term Rodent Carcinogenicity Studies of Pharmaceuticals, ICH S1A. US Food and Drug Administration. <http://www.fda.gov/cder/guidance/ichs1a.pdf> (1996).
Guidance for Industry: S1B Testing for Carcinogenicity of Pharmaceuticals. US Food and Drug Administration. <http://www.fda.gov/cder/guidance/1854fnl.pdf> (1997).
Press Release, Novo Nordisk suspends the clinical development of ragaglitazar (NN622), Bagsvaerd, Denmark (July 22, 2002).
Press Release, January 30, 2003, Pharmaceutical Research and Manufacturers of America (PhRMA), Pharmceutical Companies Receive FDA Approval for 26 NME and Biologics, 63 Other New Medicines and 172 New Uses for Medicines in 2002.
Ackerman, B. L., Murphy, A. T. & Berna, M. J. The resurgence of column switching techniques to facilitate rapid LC/MS/MS based bioanalysis in drug discovery. Amer. Pharm. Rev. 5, 54–63 (2002).
Clarke, N. J., Rindgen, D., Korfmacher, W. A. & Cox, K. A. Systematic LC/MS metabolite identification in drug discovery. Anal. Chem. 73, 430A–439A (2001).
March, R. E. An introduction to quadrupole ion trap mass spectrometry. J. Mass Spectrom. 32, 351–369 (1997).
Chernushevich, I. V., Loboda A. V. & Thomson, B. A. An introduction to quadrupole-time-of-flight mass spectrometry. J. Mass Spectrom. 36, 849–865 (2001).
Albert, K. Liquid chromatography — nuclear magnetic resonance spectroscopy. J. Chromatogr. A. 856, 199–211 (1999).
Hagar, J. W. A new linear ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 16, 512–526 (2002).
Marshall, A. G., Hendrickson, C. L. & Shi, D. -H. S. Scaling MS plateaus with FTICR MS. Anal. Chem. 74, 252A–259A (2002).
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
FURTHER INFORMATION
National Center for Toxicogenomics
US Food and Drug Administration
International Conference on Harmonization
Indiana University School of Medicine Cytochrome P450 Drug Interaction Table
Glossary
- TORSADES DE POINTES
-
This is a form of polymorphic ventricular tachycardia that is preceded by a prolongation of the QT interval. Although this condition is found in many clinical settings, it is mostly induced by drugs and drug interactions that prompt a long QT syndrome.
- MINI-PIG
-
A small species of pig weighing about 20–45kg.
- GAMMA SCINTIGRAPHY
-
Gamma scintigraphy is a non-invasive method of examining the deposition of a compound in the test subject's body using standard radiolabelling techniques. A computerized image of γ-emissions displays overall product deposition, including differentiation of test article concentration.
- POSITRON EMISSION TOMOGRAPHY
-
(PET). A method for imaging that measures changes in blood flow associated with brain function by detecting positrons emitted by radioactively labelled substances that have been injected into the body.
Rights and permissions
About this article
Cite this article
Pritchard, J., Jurima-Romet, M., Reimer, M. et al. Making Better Drugs: Decision Gates in Non-Clinical Drug Development. Nat Rev Drug Discov 2, 542–553 (2003). https://doi.org/10.1038/nrd1131
Issue Date:
DOI: https://doi.org/10.1038/nrd1131
This article is cited by
-
Mass spectrometry imaging in zebrafish larvae for assessing drug safety and metabolism
Analytical and Bioanalytical Chemistry (2021)
-
Manganese catalyst enables exploration of the magic methyl effect
Nature (2020)
-
Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles
Translational Medicine Communications (2019)
-
Genome-wide evidences of bisphenol a toxicity using Schizosaccharomyces pombe
Archives of Pharmacal Research (2018)
-
A Predictive Model for Toxicity Effects Assessment of Biotransformed Hepatic Drugs Using Iterative Sampling Method
Scientific Reports (2016)