Key Points
-
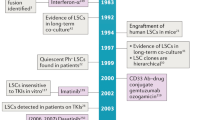
Multiple myeloma is almost always preceded by monoclonal gammopathy of undetermined significance, and at least one-quarter of all patients with myelodysplastic syndromes (MDS) have disease that evolves into acute myeloid leukaemia; in turn, MDS are frequently anteceded by clonal haematopoiesis of indeterminate potential
-
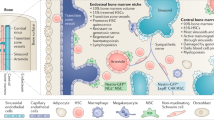
The bone-marrow microenvironment has been recognized to be a vibrant and complex living tissue that can aid and abet neoplastic disease processes
-
Neoplastic clones can transform the local bone-marrow microenvironment to favour their own growth at the expense of nonmalignant haematopoietic cells
-
An intricate and dynamic relationship between stem cell 'seeds' and the niche 'soil' helps to determine whether healthy haematopoiesis or an overgrowth of haematological malignancies occurs within the bone marrow
-
Targeting microenvironment-specific alterations might not only prevent disease progression from precursor states but also enhance the effectiveness of available therapies for the overt malignancies once progression has occurred
Abstract
Several haematological malignancies, including multiple myeloma (MM) and acute myeloid leukaemia (AML), have well-defined precursor states that precede the development of overt cancer. MM is almost always preceded by monoclonal gammopathy of undetermined significance (MGUS), and at least a quarter of all patients with myelodysplastic syndromes (MDS) have disease that evolves into AML. In turn, MDS are frequently anteceded by clonal haematopoiesis of indeterminate potential (CHIP). The acquisition of additional genetic and epigenetic alterations over time clearly influences the increasingly unstable and aggressive behaviour of neoplastic haematopoietic clones; however, perturbations in the bone-marrow microenvironment are increasingly recognized to have key roles in initiating and supporting oncogenesis. In this Review, we focus on the concept that the haematopoietic neoplasia–microenvironment relationship is an intimate rapport between two partners, provide an overview of the evidence supporting a role for the bone-marrow niche in promoting neoplasia, and discuss the potential for niche-specific therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schanz, J. et al. Coalesced multicentric analysis of 2,351 patients with myelodysplastic syndromes indicates an underestimation of poor-risk cytogenetics of myelodysplastic syndromes in the international prognostic scoring system. J. Clin. Oncol. 29, 1963–1970 (2011).
Papaemmanuil, E. et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122, 3616–3627 (2013).
Greenberg, P. L. et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120, 2454–2465 (2012).
Steensma, D. P. et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16 (2015).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Parker, J. E. et al. 'Low-risk' myelodysplastic syndrome is associated with excessive apoptosis and an increased ratio of pro- versus anti-apoptotic bcl-2-related proteins. Br. J. Haematol. 103, 1075–1082 (1998).
Mittelman, M., Oster, H. S., Hoffman, M. & Neumann, D. The lower risk MDS patient at risk of rapid progression. Leuk. Res. 34, 1551–1555 (2010).
Walter, M. J. et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 366, 1090–1098 (2012).
Enrico, A. et al. Influence of acute myeloid leukemia progression on the prognosis of 831 patients with myelodysplastic syndromes from the Argentine database. Clin. Lymphoma Myeloma Leuk. 743–752.e5 (2017).
Østgård, L. S. et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J. Clin. Oncol. 33, 3641–3649 (2015).
Lindsley, R. C. et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 125, 1367–1376 (2015).
Landgren, O. et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 113, 5412–5417 (2009).
Weiss, B. M., Abadie, J., Verma, P., Howard, R. S. & Kuehl, W. M. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood 113, 5418–5422 (2009).
Landgren, O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematol. Am. Soc. Hematol. Educ. Program 2013, 478–487 (2013).
Kyle, R. A. & Rajkumar, S. V. Multiple myeloma. Blood 111, 2962–2972 (2008).
Rajkumar, S. V. Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 86, 57–65 (2011).
Lopez-Corral, L. et al. SNP-based mapping arrays reveal high genomic complexity in monoclonal gammopathies, from MGUS to myeloma status. Leukemia 26, 2521–2529 (2012).
Cogle, C. R. et al. Bone marrow niche in the myelodysplastic syndromes. Leuk. Res. 39, 1020–1027 (2015).
Bulycheva, E. et al. Myelodysplasia is in the niche: novel concepts and emerging therapies. Leukemia 29, 259–268 (2015).
Calvi, L. M. & Link, D. C. The hematopoietic stem cell niche in homeostasis and disease. Blood 126, 2443–2451 (2015).
Mies, A., Bulycheva, E., Rogulj, I. M., Hofbauer, L. C. & Platzbecker, U. Alterations within the osteo-hematopoietic niche in MDS and their therapeutic implications. Curr. Pharm. Des. 22, 2323–2332 (2016).
Raza, A., Cruz, R., Latif, T., Mukherjee, S. & Galili, N. The biology of myelodysplastic syndromes: unity despite heterogeneity. Hematol. Rep. 2, e4 (2010).
Roccaro, A. M. et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Invest. 123, 1542–1555 (2013).
Moschetta, M. et al. Role of endothelial progenitor cells in cancer progression. Biochim. Biophys. Acta 1846, 26–39 (2014).
Muz, B., de la Puente, P., Azab, F., Ghobrial, I. M. & Azab, A. K. Hypoxia promotes dissemination and colonization in new bone marrow niches in Waldenstrom's macroglobulinemia. Mol. Cancer Res. 13, 263–272 (2015).
Reagan, M. R. et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood 124, 3250–3259 (2014).
Roccaro, A. M. et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 9, 118–128 (2014).
Zingone, A. et al. Altered cytokine and chemokine profiles in multiple myeloma and precursor disease. Cytokine 69, 294–297 (2014).
Kawano, Y. et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 263, 160–172 (2015).
Roccaro, A. M. et al. CXCR4 regulates extra-medullary myeloma through epithelial-mesenchymal-transition-like transcriptional activation. Cell Rep. 12, 622–635 (2015).
Moschetta, M. et al. Targeting vasculogenesis to prevent progression in multiple myeloma. Leukemia 30, 1103–1115 (2016).
Sacco, A. et al. Cancer cell dissemination and homing to the bone marrow in a zebrafish model. Cancer Res. 76, 463–471 (2016).
Kawano, Y., Roccaro, A. M., Azzi, J. & Ghobrial, I. M. Multiple myeloma and the immune microenvironment. Curr. Cancer Drug Targets 17, 806–818 (2017).
Das, R. et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat. Med. 22, 1351–1357 (2016).
Schepers, K., Campbell, T. B. & Passegue, E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell 16, 254–267 (2015).
Sperling, A. S., Gibson, C. J. & Ebert, B. L. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat. Rev. Cancer 17, 5–19 (2017).
Park, D., Sykes, D. B. & Scadden, D. T. The hematopoietic stem cell niche. Front. Biosci. 17, 30–39 (2012).
Papayannopoulou, T. & Scadden, D. T. Stem-cell ecology and stem cells in motion. Blood 111, 3923–3930 (2008).
Yu, V. W. & Scadden, D. T. Hematopoietic stem cell and its bone marrow niche. Curr. Top. Dev. Biol. 118, 21–44 (2016).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Bhowmick, N. A. et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004).
Walkley, C. R., Shea, J. M., Sims, N. A., Purton, L. E. & Orkin, S. H. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081–1095 (2007).
Yang, F. C. et al. Nf1-dependent tumors require a microenvironment containing Nf1+/− and c-kit-dependent bone marrow. Cell 135, 437–448 (2008).
Raaijmakers, M. H. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857 (2010).
Kode, A. et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 506, 240–244 (2014).
Kode, A. et al. FoxO1-dependent induction of acute myeloid leukemia by osteoblasts in mice. Leukemia 30, 1–13 (2016).
Roodman, G. D. Role of the bone marrow microenvironment in multiple myeloma. J. Bone Miner. Res. 17, 1921–1925 (2002).
Podar, K., Richardson, P. G., Hideshima, T., Chauhan, D. & Anderson, K. C. The malignant clone and the bone-marrow environment. Best Pract. Res. Clin. Haematol. 20, 597–612 (2007).
Podar, K., Hideshima, T., Chauhan, D. & Anderson, K. C. Targeting signalling pathways for the treatment of multiple myeloma. Expert Opin. Ther. Targets 9, 359–381 (2005).
Podar, K., Chauhan, D. & Anderson, K. C. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 23, 10–24 (2009).
Reagan, M. R. & Ghobrial, I. M. Multiple myeloma mesenchymal stem cells: characterization, origin, and tumor-promoting effects. Clin. Cancer Res. 18, 342–349 (2012).
Manier, S. et al. Prognostic role of circulating exosomal miRNAs in multiple myeloma. Blood 129, 2429–2436 (2017).
Wang, J. & Xiao, Z. Mesenchymal stem cells in pathogenesis of myelodysplastic syndromes. Stem Cell. Investig. 1, 16 (2014).
Aanei, C. M. et al. Focal adhesion protein abnormalities in myelodysplastic mesenchymal stromal cells. Exp. Cell Res. 317, 2616–2629 (2011).
Aanei, C. M. et al. Intrinsic growth deficiencies of mesenchymal stromal cells in myelodysplastic syndromes. Stem Cells Dev. 21, 1604–1615 (2012).
Geyh, S. et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia 27, 1841–1851 (2013).
Blau, O. et al. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood 118, 5583–5592 (2011).
Blau, O. et al. Chromosomal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp. Hematol. 35, 221–229 (2007).
Oliveira, F. M. et al. Differential expression of AURKA and AURKB genes in bone marrow stromal mesenchymal cells of myelodysplastic syndrome: correlation with G-banding analysis and FISH. Exp. Hematol. 41, 198–208 (2013).
Borojevic, R. et al. Bone marrow stroma in childhood myelodysplastic syndrome: composition, ability to sustain hematopoiesis in vitro, and altered gene expression. Leuk. Res 28, 831–844 (2004).
Li, X. & Deeg, H. J. Murine xenogeneic models of myelodysplastic syndrome: an essential role for stroma cells. Exp. Hematol. 42, 4–10 (2014).
Medyouf, H. et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell 14, 824–837 (2014).
Todoerti, K. et al. Distinct transcriptional profiles characterize bone microenvironment mesenchymal cells rather than osteoblasts in relationship with multiple myeloma bone disease. Exp. Hematol. 38, 141–153 (2010).
Garayoa, M. et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia 23, 1515–1527 (2009).
Flores-Figueroa, E., Arana-Trejo, R. M., Gutiérrez-Espíndola, G., Pérez-Cabrera, A. & Mayani, H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk. Res. 29, 215–224 (2005).
Santamaria, C. et al. Impaired expression of DICER, DROSHA, SBDS and some microRNAs in mesenchymal stromal cells from myelodysplastic syndrome patients. Haematologica 97, 1218–1224 (2012).
Zambetti, N. A. et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell 19, 613–627 (2016).
Olechnowicz, S. W. & Edwards, C. M. Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res. 74, 1625–1631 (2014).
Yavropoulou, M. P. & Yovos, J. G. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones 6, 279–294 (2007).
Roodman, G. D. Pathogenesis of myeloma bone disease. Leukemia 23, 435–441 (2009).
Roodman, G. D. New potential targets for treating myeloma bone disease. Clin. Cancer Res. 12, 6270s–6273s (2006).
Toscani, D., Bolzoni, M., Accardi, F., Aversa, F. & Giuliani, N. The osteoblastic niche in the context of multiple myeloma. Ann. NY Acad. Sci. 1335, 45–62 (2015).
Bianchi, G. & Munshi, N. C. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood 125, 3049–3058 (2015).
Huston, A. & Roodman, G. D. Role of the microenvironment in multiple myeloma bone disease. Future Oncol. 2, 371–378 (2006).
Tripodo, C. et al. Stromal SPARC contributes to the detrimental fibrotic changes associated with myeloproliferation whereas its deficiency favors myeloid cell expansion. Blood 120, 3541–3554 (2012).
Balderman, S. R. et al. Targeting of the bone marrow microenvironment improves outcome in a murine model of myelodysplastic syndrome. Blood 127, 616–625 (2016).
Kitagawa, M. et al. Overexpression of tumor necrosis factor (TNF)-alpha and interferon (IFN)-gamma by bone marrow cells from patients with myelodysplastic syndromes. Leukemia 11, 2049–2054 (1997).
Wang, Z. et al. The different immunoregulatory functions on dendritic cells between mesenchymal stem cells derived from bone marrow of patients with low-risk or high-risk myelodysplastic syndromes. PLoS ONE 8, e57470 (2013).
Allampallam, K. et al. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. Int. J. Hematol. 75, 289–297 (2002).
Feng, X. et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica 96, 602–606 (2011).
Weiss, G. & Goodnough, L. T. Anemia of chronic disease. N. Engl. J. Med. 352, 1011–1023 (2005).
Qiang, Y. W., Kopantzev, E. & Rudikoff, S. Insulinlike growth factor-I signaling in multiple myeloma: downstream elements, functional correlates, and pathway cross-talk. Blood 99, 4138–4146 (2002).
Alsayed, Y. et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 109, 2708–2717 (2007).
Dankbar, B. et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood 95, 2630–2636 (2000).
Chauhan, D. et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J. Biol. Chem. 275, 27845–27850 (2000).
Jacamo, R. et al. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-kappaB mediates chemoresistance. Blood 123, 2691–2702 (2014).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Swann, J. B. & Smyth, M. J. Immune surveillance of tumors. J. Clin. Invest. 117, 1137–1146 (2007).
Coulson-Thomas, V. J., Coulson-Thomas, Y. M., Gesteira, T. F. & Kao, W. W. Extrinsic and intrinsic mechanisms by which mesenchymal stem cells suppress the immune system. Ocul. Surf. 14, 121–134 (2016).
Uccelli, A. & de Rosbo, N. K. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann. NY Acad. Sci. 1351, 114–126 (2015).
Ghannam, S., Pene, J., Moquet-Torcy, G., Jorgensen, C. & Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 185, 302–312 (2010).
Ghannam, S., Bouffi, C., Djouad, F., Jorgensen, C. & Noel, D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res. Ther. 1, 2 (2010).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Noonan, K. et al. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood 116, 3554–3563 (2010).
Feyler, S. et al. CD4+CD25+FoxP3+ regulatory T cells are increased whilst CD3+CD4−CD8−αβTCR+ double negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br. J. Haematol. 144, 686–695 (2009).
Zelle-Rieser, C. et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J. Hematol. Oncol. 9, 116 (2016).
Giuliani, N. et al. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood 100, 4615–4621 (2002).
Roussou, M. et al. Increased expression of macrophage inflammatory protein-1α on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 23, 2177–2181 (2009).
Gorgun, G. T. et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 121, 2975–2987 (2013).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Kawano, Y. et al. Characterization of the role of regulatory T cells (Tregs) in inducing progression of multiple myeloma. Blood 126, 502 (2015).
Sallman, D. A., Cluzeau, T., Basiorka, A. A. & List, A. Unraveling the pathogenesis of MDS: the NLRP3 inflammasome and pyroptosis drive the MDS phenotype. Front. Oncol. 6, 151 (2016).
Basiorka, A. A. et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood 128, 2960–2975 (2016).
Xin, J. et al. Necroptosis in spontaneously-mutated hematopoietic cells induces autoimmune bone marrow failure in mice. Haematologica 102, 295–307 (2017).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Testa, U., Labbaye, C., Castelli, G. & Pelosi, E. Oxidative stress and hypoxia in normal and leukemic stem cells. Exp. Hematol. 44, 540–560 (2016).
Laukka, T. et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J. Biol. Chem. 291, 4256–4265 (2016).
Passaro, D. et al. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell 32, 324–341 (2017).
Teofili, L. et al. Endothelial progenitor cell dysfunction in myelodysplastic syndromes: possible contribution of a defective vascular niche to myelodysplasia. Neoplasia 17, 401–409 (2015).
Colla, S. et al. Low bone marrow oxygen tension and hypoxia-inducible factor-1α overexpression characterize patients with multiple myeloma: role on the transcriptional and proangiogenic profiles of CD138+ cells. Leukemia 24, 1967–1970 (2010).
Azab, A. K. et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 119, 5782–5794 (2012).
Glavey, S. V. et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia 31, 2426–2434 (2017).
Yang, Y. et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood 100, 610–617 (2002).
Foroushani, A. et al. Large-scale gene network analysis reveals the significance of extracellular matrix pathway and homeobox genes in acute myeloid leukemia: an introduction to the Pigengene package and its applications. BMC Med. Genom. 10, 16 (2017).
Mateos, M. V. et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N. Engl. J. Med. 369, 438–447 (2013).
Zorat, F. et al. The clinical and biological effects of thalidomide in patients with myelodysplastic syndromes. Br. J. Haematol. 115, 881–894 (2001).
Aguayo, A., Giles, F. & Albitar, M. Vascularity, angiogenesis and angiogenic factors in leukemias and myelodysplastic syndromes. Leuk. Lymphoma 44, 213–222 (2003).
Ebert, B. L. et al. An erythroid differentiation signature predicts response to lenalidomide in myelodysplastic syndrome. PLoS Med. 5, e35 (2008).
Lu, L. et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc. Res. 77, 78–86 (2009).
Kronke, J. et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 523, 183–188 (2015).
Kronke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014).
Oliva, E. N. et al. Lenalidomide in International Prognostic Scoring System Low and Intermediate-1 risk myelodysplastic syndromes with del(5q): an Italian phase II trial of health-related quality of life, safety and efficacy. Leuk. Lymphoma 54, 2458–2465 (2013).
Wang, E. S. et al. A randomized, double-blind, placebo-controlled phase 2 study evaluating the efficacy and safety of romiplostim treatment of patients with low or intermediate-1 risk myelodysplastic syndrome receiving lenalidomide. J. Hematol. Oncol. 5, 71 (2012).
Swami, A. et al. Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc. Natl Acad. Sci. USA 111, 10287–10292 (2014).
Rajkumar, S. V. & Kyle, R. A. Angiogenesis in multiple myeloma. Semin. Oncol. 28, 560–564 (2001).
Palumbo, A. et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 375, 754–766 (2016).
Dimopoulos, M. A. et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 375, 1319–1331 (2016).
Lonial, S. et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 373, 621–631 (2015).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Akbay, E. A. et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 3, 1355–1363 (2013).
Benson, D. M. Jr et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010).
Rosenblatt, J. et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J. Immunother. 34, 409–418 (2011).
Atanackovic, D., Luetkens, T. & Kroger, N. Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia 28, 993–1000 (2014).
Badros, A. et al. Pembrolizumab, pomalidomide, and low-dose dexamethasone for relapsed/refractory multiple myeloma. Blood 130, 1189–1197 (2017).
Ayed, A. O., Chang, L. J. & Moreb, J. S. Immunotherapy for multiple myeloma: current status and future directions. Crit. Rev. Oncol. Hematol. 96, 399–412 (2015).
Cheah, C. Y., Fowler, N. H. & Neelapu, S. S. Targeting the programmed death-1/programmed death-ligand 1 axis in lymphoma. Curr. Opin. Oncol. 27, 384–391 (2015).
Jelinek, T. & Hajek, R. PD-1/PD-L1 inhibitors in multiple myeloma: the present and the future. Oncoimmunology 5, e1254856 (2016).
[No authors listed] FDA alerts healthcare professionals and oncology clinical investigators about two clinical trials on hold evaluating KEYTRUDA® (pembrolizumab) in patients with multiple myeloma. US Food and Drug Administration https://www.fda.gov/Drugs/DrugSafety/ucm574305.htm (2017).
Agazzi, A. Report on the 56th ASH Annual Meeting, San Francisco, 4–9 December 2014. ecancermedicalscience 9, 514 (2015).
Atanackovic, D., Radhakrishnan, S. V., Bhardwaj, N. & Luetkens, T. Chimeric antigen receptor (CAR) therapy for multiple myeloma. Br. J. Haematol. 172, 685–698 (2016).
Abe-Suzuki, S. et al. CXCL12+ stromal cells as bone marrow niche for CD34+ hematopoietic cells and their association with disease progression in myelodysplastic syndromes. Lab. Invest. 94, 1212–1223 (2014).
Zhang, Y. et al. SDF-1/CXCR4 axis in myelodysplastic syndromes: correlation with angiogenesis and apoptosis. Leuk. Res. 36, 281–286 (2012).
Sison, E. A., McIntyre, E., Magoon, D. & Brown, P. Dynamic chemotherapy-induced upregulation of CXCR4 expression: a mechanism of therapeutic resistance in pediatric AML. Mol. Cancer Res. 11, 1004–1016 (2013).
Ludwig, H. et al. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib dexamethasone in relapsed/refractory multiple myeloma: a phase IIa study. Leukemia 31, 997–1000 (2017).
Uy, G. L. et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 119, 3917–3924 (2012).
Morgan, G. J. et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol. 12, 743–752 (2011).
D'Arena, G. et al. Pamidronate versus observation in asymptomatic myeloma: final results with long-term follow-up of a randomized study. Leuk. Lymphoma 52, 771–775 (2011).
Dong, M. et al. Phase III clinical study of zoledronic acid in the treatment of pain induced by bone metastasis from solid tumor or multiple myeloma [Chinese]. Zhonghua Zhong Liu Za Zhi 30, 215–220 (2008).
Rosen, L. S. et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 98, 1735–1744 (2003).
Musto, P. et al. Pamidronate reduces skeletal events but does not improve progression-free survival in early-stage untreated myeloma: results of a randomized trial. Leuk. Lymphoma 44, 1545–1548 (2003).
Iyer, S. P. et al. A phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br. J. Haematol. 167, 366–375 (2014).
Qian, J. et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood 110, 1587–1594 (2007).
Vij, R. et al. An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. Am. J. Hematol. 84, 650–656 (2009).
Ghobrial, I. M. & Landgren, O. How I treat smoldering multiple myeloma. Blood 124, 3380–3388 (2014).
Stasi, R. & Amadori, S. Infliximab chimaeric anti-tumour necrosis factor alpha monoclonal antibody treatment for patients with myelodysplastic syndromes. Br. J. Haematol. 116, 334–337 (2002).
Scott, B. L. et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br. J. Haematol. 149, 706–710 (2010).
Scott, B. L. et al. Prolonged responses in patients with MDS and CMML treated with azacitidine and etanercept. Br. J. Haematol. 148, 944–947 (2010).
Garcia-Manero, G. et al. A phase 2, randomized, double-blind, multicenter study comparing siltuximab plus best supportive care (BSC) with placebo plus BSC in anemic patients with International Prognostic Scoring System low- or intermediate-1-risk myelodysplastic syndrome. Am. J. Hematol. 89, E156–E162 (2014).
Magarotto, V., Salvini, M., Bonello, F., Bringhen, S. & Palumbo, A. Strategy for the treatment of multiple myeloma utilizing monoclonal antibodies: a new era begins. Leuk. Lymphoma 57, 537–556 (2016).
Orlowski, R. Z. et al. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 90, 42–49 (2015).
Shah, J. J. et al. Siltuximab (CNTO 328) with lenalidomide, bortezomib and dexamethasone in newly-diagnosed, previously untreated multiple myeloma: an open-label phase I trial. Blood Cancer J. 6, e396 (2016).
van Rhee, F. et al. A phase 2, open-label, multicenter study of the long-term safety of siltuximab (an anti-interleukin-6 monoclonal antibody) in patients with multicentric Castleman disease. Oncotarget 6, 30408–30419 (2015).
Carrancio, S. et al. An activin receptor IIA ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cells and haemoglobin. Br. J. Haematol. 165, 870–882 (2014).
Giagounidis, A. et al. Luspatercept treatment leads to long term increases in hemoglobin and reductions in transfusion burden in patients with low or intermediate-1 risk myelodysplastic syndromes (MDS): preliminary results from the phase 2 PACE-MDS extension study. Blood 126, 92 (2015).
Dussiot, M. et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nat. Med. 20, 398–407 (2014).
Mies, A. & Platzbecker, U. Increasing the effectiveness of hematopoiesis in myelodysplastic syndromes: erythropoiesis-stimulating agents and transforming growth factor-beta superfamily inhibitors. Semin. Hematol. 54, 141–146 (2017).
McMillin, D. W. et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat. Med. 16, 483–489 (2010).
Straussman, R. et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487, 500–504 (2012).
Yaccoby, S. et al. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br. J. Haematol. 116, 278–290 (2002).
Wang, J. et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 124, 555–566 (2014).
Tai, Y. T. et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28, 155–165 (2014).
Roccaro, M. et al. Stroma-derived exosomes mediate oncogenesis in multiple myeloma Blood 118, 625 (2011).
Maiso, P. et al. Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res. 75, 2071–2082 (2015).
Hu, J. et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood 116, 1524–1527 (2010).
Laubach, J. et al. Preliminary safety and efficacy of TH-302, an investigational hypoxia-activated prodrug, combined with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma (RR MM) [abstract]. J. Clin. Oncol. 32 (Suppl.), 8534 (2014).
Bajaj, J. et al. CD98-mediated adhesive signaling enables the establishment and propagation of acute myelogenous leukemia. Cancer Cell 30, 792–805 (2016).
Carter, B. Z. et al. Anti-apoptotic ARC protein confers chemoresistance by controlling leukemia-microenvironment interactions through a NFκB/IL1β signaling network. Oncotarget 7, 20054–20067 (2016).
Stone, R. M. et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 377, 454–464 (2017).
Ghiaur, G. & Levis, M. Mechanisms of Resistance to FLT3 Inhibitors and the Role of the Bone Marrow Microenvironment. Hematol. Oncol. Clin. North Am. 31, 681–692 (2017).
Verstovsek, S. et al. Clinical relevance of vascular endothelial growth factor receptors 1 and 2 in acute myeloid leukaemia and myelodysplastic syndrome. Br. J. Haematol. 118, 151–156 (2002).
Hu, Q. et al. Soluble vascular endothelial growth factor receptor 1, and not receptor 2, is an independent prognostic factor in acute myeloid leukemia and myelodysplastic syndromes. Cancer 100, 1884–1891 (2004).
Verburgh, E. et al. Additional prognostic value of bone marrow histology in patients subclassified according to the International Prognostic Scoring System for myelodysplastic syndromes. J. Clin. Oncol. 21, 273–282 (2003).
Stoddart, A. et al. Inhibition of WNT signaling in the bone marrow niche prevents the development of MDS in the Apcdel/+ MDS mouse model. Blood 129, 2959–2970 (2017).
Shastri, A., Will, B., Steidl, U. & Verma, A. Stem and progenitor cell alterations in myelodysplastic syndromes. Blood 129, 1586–1594 (2017).
Chen, X. et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J. Clin. Invest. 123, 4595–4611 (2013).
Chauhan, D. et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell 16, 309–323 (2009).
Ray, A. et al. A novel agent SL-401 induces anti-myeloma activity by targeting plasmacytoid dendritic cells, osteoclastogenesis and cancer stem-like cells. Leukemia 31, 2652–2660 (2017).
Vercauteren, S. M. et al. T cells of patients with myelodysplastic syndrome are frequently derived from the malignant clone. Br. J. Haematol. 156, 409–412 (2012).
Epling-Burnette, P. K. et al. Prevalence and clinical association of clonal T-cell expansions in myelodysplastic syndrome. Leukemia 21, 659–667 (2007).
Singhal, S. et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 341, 1565–1571 (1999).
Weber, D. et al. Lenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): results of a North American phase III study (MM-009) [abstract]. J. Clin. Oncol. 24, (Suppl.), 7521 (2006).
Dimopoulos, M. et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N. Engl. J. Med. 357, 2123–2132 (2007).
Lonial, S. et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J. Clin. Oncol. 30, 1953–1959 (2012).
Lesokhin, A. A. et al. Preliminary results of a phase I study of nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies. Blood 124, 291 (2014).
Sidaway, P. Haematological cancer: Pembrolizumab is effective in multiple myeloma. Nat. Rev. Clin. Oncol. 14, 393 (2017).
Davids, M. S. et al. Ipilimumab for patients with relapse after allogeneic transplantation. N. Engl. J. Med. 375, 143–153 (2016).
Noonan, K. A. et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci. Transl Med. 7, 288ra78 (2015).
Pyzer, A. R., Avigan, D. E. & Rosenblatt, J. Clinical trials of dendritic cell-based cancer vaccines in hematologic malignancies. Hum. Vaccin. Immunother. 10, 3125–3131 (2014).
Rosenblatt, J. et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin. Cancer Res. 19, 3640–3648 (2013).
Ghobrial, I. et al. Phase I trial of plerixafor and bortezomib as a chemosensitization strategy in relapsed or relapsed/refractory multiple myeloma. Blood 118, 1874 (2011).
Acknowledgements
I.M.G. acknowledges research funding from the US Department of Health & Human Services, National Institutes of Health, Center for Scientific Review (PQ1 grant 1R01CA205954-01). Editorial assistance was provided by Helen Pickersgil at Lifescience.
Author information
Authors and Affiliations
Contributions
All authors researched data, wrote, and reviewed and/or edited the manuscript before submission. Additionally, I.M.G., K.C.A, and D.P.S. made substantial contributions to discussion of content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Ghobrial, I., Detappe, A., Anderson, K. et al. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol 15, 219–233 (2018). https://doi.org/10.1038/nrclinonc.2017.197
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.197
This article is cited by
-
Development and application of nanomaterials, nanotechnology and nanomedicine for treating hematological malignancies
Journal of Hematology & Oncology (2023)
-
A novel prognostic model based on pyroptosis-related genes for multiple myeloma
BMC Medical Genomics (2023)
-
P2X1 enhances leukemogenesis through PBX3-BCAT1 pathways
Leukemia (2023)
-
Immune dysregulation in multiple myeloma: the current and future role of cell-based immunotherapy
International Journal of Hematology (2023)
-
Monocytosis and Multiple Myeloma: treatment-related acute leukaemia?
Surgical and Experimental Pathology (2022)